
Glutamine dependence and PIK3CA mutations in colon-rectal cancer cells: ATF4 enters the stage
Capacity to reprogram the metabolic activities exhibited by cancer cells distinguishes tumors from normal tissues supporting their unregulated growth. While alterations in glucose metabolism were the first cancer-related metabolic anomalies to be detected and extensively investigated, in the last years an increasing number of studies has progressively unveiled the role that the amino acid glutamine plays in an enlarging group of human tumors. Glutamine, as a potential donor of either carbon or nitrogen, plays several metabolic roles. Some of these are ubiquitous, others strictly tissue specific (1). In selected cancer types, glutamine is avidly transported from the extracellular compartment, and its metabolism is up-regulated to provide energy production and sustain biosynthetic aims. Cancers that exhibit particularly high requirements for glutamine are named glutamine-addicted (2). A variety of oncogenes and tumor suppressor genes impact glutamine metabolism in cancer cells (1) including Myc overexpression (1), p53 (3), Rb tumor suppressor (4) and Von Hippel-Lindau (VHL) suppressor through hypoxia-inducible factor (HIF)-1 (5). Nevertheless, the mechanism by which many cancer cells become dependent on glutamine is still under investigation.
Recently, Hao et al. (6) have shown that PIK3CA mutations enhance and reprogram glutamine metabolism by inducing glutamate pyruvate transaminase 2 (GPT2), also known as ALT2, in colon-rectal cancer cells (CRCs) suggesting that this mutation could be responsible for their glutamine dependence. PIK3CA codes for p110α, the catalytic subunit of phosphatidylinositol 3-kinase α (PI3Kα), which, upon an activating interaction with one of several regulatory subunits, synthesizes phosphatidylinositol-3,4,5-trisphosphate (PIP3) from phosphatidylinositol-4,5-bisphosphate (PIP2). Promoting the phosphorylation of AKT through 3-phosphoinositide dependent protein kinase 1 (PDK1), PIP3 constitutes a key signal for cell survival and proliferation. The importance of the findings reported by Hao et al. (6) is evident, considering that PIK3CA mutations may be present in up to 30% of colon cancers (7) and represent one of the most common oncogene alterations in human tumors (8). In spite of the high frequency of these mutations any association between PIK3CA mutations and alterations in glutamine metabolism had been not reported thus far. Similarly to that observed in several glutamine-dependent cancer models, also in PIK3CA-mutated CRCs, glutamine is rapidly converted into α-ketoglutarate and used for anaplerosis, thus ensuring compatibility of optimal Krebs cycle function for ATP production with large availability of biosynthetic precursors needed for rapid proliferation. While anaplerosis is commonly considered one of the most important metabolic roles of glutamine in tumor cells, it should be considered that maintenance of the intracellular pool of α-ketoglutarate may have additional relevance. Indeed, this metabolite is an obliged substrate or co-factor of enzymes, such as prolyl hydroxylases and 5-methylcytosine hydroxylase, which modify proteins and DNA and are involved in important regulatory mechanisms of gene expression. What differentiates the models studied by Hao et al. (6) to previously described glutamine-addicted tumors is that PIK3CA mutations do not influence the expression of GLS (glutaminase), GLS2 (glutaminase 2) or GDH (glutamate dehydrogenase), the enzymes found more commonly overexpressed in glutamine-dependent cancers. Interestingly, Hao et al. (6) show that, instead, the PIK3CA target responsible for the increased glutamine metabolisms in PIK3CA mutated cells is GPT2, the mitochondrial isoform of glutamate pyruvate transaminase. PIK3CA mutated CRCs express significantly more GPT2 than WT counterparts, and, importantly, this finding is confirmed comparing 10 tumors with PIK3CA mutations and 10 tumors without mutations in the PI3Kα pathway.
The pivotal role of GPT2 in the development of neoplastic phenotype of the models studied by Hao et al. (6) is demonstrated by the severe delay in tumor growth observed if GPT2 is silenced or inhibited by aminooxyacetate (AOA). Changes in enzyme expression in cancer cells have been studied less in depth for transaminases than other enzymes involved in Gln metabolism, and the studies available usually concern glutamic-oxaloacetic transaminase (GOT) rather than GPT (9-11). Recently, however, Korangath et al. (12) observed a net GPT2 overexpression in glutamine-dependent breast cancer cells, but increased expression of other enzymes involved in the metabolism of the amino acids (GOT1/2, GLS2) were also present in the same models. Weinberg et al. (13) reported that K-RAS-dependent tumorigenesis in HCT116 CRCs, one of the models studied by Hao et al. (6), required GPT2 activity. However, they did not investigate changes in GPT2 expression and, on the other hand, Hao et al. (6) convincingly demonstrate that the effect of PIK3CA mutation on GPT2 is K-RAS independent. In other glutamine dependent tumors (14-16), ASCT2, one of the membrane carriers that transport glutamine, is overexpressed and cancer cell growth is hindered inhibiting its activity or expression. Because of the lack of ASCT2 overexpression in PIK3CA-mutated cells, this approach may not be useful in this model (6). However, since these cells exhibit significantly increased glutamine consumption, they should also take up more amino acid. This issue should prompt the assessment of glutamine transport in PIK3CA-mutated cells and the possible involvement of other glutamine transporters.
Another original finding reported by Hao et al. (6) is the characterization of the transduction pathway that links PIK3CA mutations and GPT2 overexpression (see Figure 1). Indeed, while AKT, the most well studied target of PIP3-activated PDK1, is obviously activated in PIK3CA mutated cells, it does not seem to be responsible for GPT2 up-regulation. In contrast, Hao et al. (6) elegantly demonstrate that PIK3CA mutations induce GPT2 through an AKT-independent transduction axis, consisting of the increased expression of the transcription factor ATF4, promoted by the activity of the RSK2 kinase, another substrate of PDK1. This novel mechanism is very interesting, because it links metabolic alterations in cancer cells and the endoplasmic reticulum (ER) stress pathway. Indeed, the overexpression of the transcription factor ATF4 is one of the key mechanisms involved in the exertion of ER stress by cells undergoing various types of stress. However, while in stressed cells ATF4 overexpression is usually due to its increased CAP-independent synthesis, in PIK3CA mutated CRCs the half-life of the protein increases due to its RSK2-dependent phosphorylation in S245. Hao et al. (6) go further, demonstrating that p-ATF4 has an increased interaction with the deubiquitinase USP8 and, hence, is preserved from proteosomal degradation. However, it should be stressed that, even in the models studied by Hao et al. (6), AKT activation remains important, for instance, to promote the metastatic behavior (17).
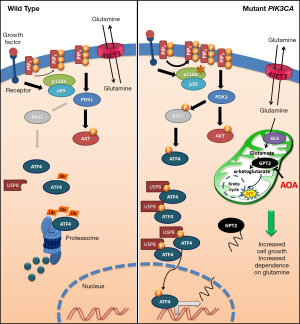
The main findings reported in the manuscript by Hao et al. (6) are summarized in Figure 1 highlighting the different behavior between PIK3CA mutated and not mutated CRCs in terms of glutamine dependence and, consequently, in cell growth.
Besides its clear relevance in basic cancer biology, the study of Hao et al. (6) has evident translational implications. First of all, PIK3CA mutations promote the rapid growth of CRCs, as demonstrated both in vitro and in vivo, but also render them more sensitive to glutamine deprivation. Thus, while the pathway described by Hao et al. (6) confers a clear-cut proliferative advantage, it also constitutes the basis for metabolic fragility. This apparently paradoxical observation constitutes the rationale for therapeutic approaches based on interference with glutamine metabolism. Although the prognostic role of PIK3CA on colon cancer is complex and, possibly, depends on the tumor site and the mutation(s) present (18), the possibility that the suppression of GPT2 activity phenotypically limits the consequences of PIK3CA mutations is of evident interest. Second, the efficacy and relative safeness exhibited by AOA suggest the feasibility of therapeutic approaches based on the inhibition of GPT2. AOA has been already used to hinder the growth of tumors, such as breast cancer (12,19), where GPT2 was not the sole enzyme involved in the cancer associated metabolic alterations. In fact, as underlined by Hao et al., AOA is not specific, since it inhibits not only the other transaminases but, more generally, all pyridoxal phosphate-dependent enzymes involved in amino acid metabolism. Although the evidence that AOA is not effective in GPT2-silenced cells demonstrates that in the models studied by Hao et al. (6) its effect is likely due to GPT2 inhibition, GPT2-specific inhibitors would be preferable in order to avoid or reduce possible side effects. Interestingly, in both colon cancer (6) and breast cancer (19) xenografts AOA effects are more evident in vivo than in vitro. This discrepancy has been also observed in hepatocellular carcinoma xenografts with a different glutamine-targeting approach (20). As Hao et al. (6) underline, these data would suggest that CRCs are more dependent on glutamine in the in vivo microenvironment than in the in vitro tissue culture conditions.
Finally, the possibility that the mechanism described by Hao et al. (6) is not exclusive for colon cancer is intriguing. In particular, its presence in other tumors where PIK3CA mutations are frequently detected, such as brain and gastric cancers (7), should be assessed in the future.
Acknowledgments
Funding: This work was supported in part by a grant from the Associazione Italiana per la Ricerca sul Cancro (AIRC) Investigator Grant (IG) 2014 15531 (N Giuliani), and a fellowship Fondazione Italiana per la Ricerca sul Cancro 18152 (M Bolzoni).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Fengbo Tan, MD (Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, Changsha, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest 2013;123:3678-84. [Crossref] [PubMed]
- Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 2010;35:427-33. [Crossref] [PubMed]
- Suzuki S, Tanaka T, Poyurovsky MV, et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci U S A 2010;107:7461-6. [Crossref] [PubMed]
- Reynolds MR, Lane AN, Robertson B, et al. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene 2014;33:556-66. [Crossref] [PubMed]
- Gameiro PA, Yang J, Metelo AM, et al. In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab 2013;17:372-85. [Crossref] [PubMed]
- Hao Y, Samuels Y, Li Q, et al. Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer. Nat Commun 2016;7:11971. [Crossref] [PubMed]
- Samuels Y, Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle 2004;3:1221-4. [Crossref] [PubMed]
- Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 2014;505:495-501. [Crossref] [PubMed]
- Son J, Lyssiotis CA, Ying H, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013;496:101-5. [Crossref] [PubMed]
- Qing G, Li B, Vu A, et al. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell 2012;22:631-44. [Crossref] [PubMed]
- Yang Y. Enhancing doxorubicin efficacy through inhibition of aspartate transaminase in triple-negative breast cancer cells. Biochem Biophys Res Commun 2016;473:1295-300. [Crossref] [PubMed]
- Korangath P, Teo WW, Sadik H, et al. Targeting Glutamine Metabolism in Breast Cancer with Aminooxyacetate. Clin Cancer Res 2015;21:3263-73. [Crossref] [PubMed]
- Weinberg F, Hamanaka R, Wheaton WW, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A 2010;107:8788-93. [Crossref] [PubMed]
- van Geldermalsen M, Wang Q, Nagarajah R, et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 2016;35:3201-8. [Crossref] [PubMed]
- Bolzoni M, Chiu M, Accardi F, et al. Dependence on glutamine uptake and glutamine addiction characterize myeloma cells: a new attractive target. Blood 2016;128:667-79. [Crossref] [PubMed]
- Wang Q, Hardie RA, Hoy AJ, et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J Pathol 2015;236:278-89. [Crossref] [PubMed]
- Ericson K, Gan C, Cheong I, et al. Genetic inactivation of AKT1, AKT2, and PDPK1 in human colorectal cancer cells clarifies their roles in tumor growth regulation. Proc Natl Acad Sci U S A 2010;107:2598-603. [Crossref] [PubMed]
- Liao X, Morikawa T, Lochhead P, et al. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clin Cancer Res 2012;18:2257-68. [Crossref] [PubMed]
- Thornburg JM, Nelson KK, Clem BF, et al. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res 2008;10:R84. [Crossref] [PubMed]
- Chiu M, Tardito S, Pillozzi S, et al. Glutamine depletion by crisantaspase hinders the growth of human hepatocellular carcinoma xenografts. Br J Cancer 2014;111:1159-67. [Crossref] [PubMed]


