Liver resection of metastases for colorectal cancer, gastric cancer and breast cancer: two hospital experiences
Introduction
Liver metastases mean terminal stage of most malignant tumors. Under the circumstances, some doctors tend to be conservative, while some doctors tend to be positive. Along with the progress of medical technology and evidence based medical science, more and more doctors believe that aggressive treatment including surgical interventions may give rise to better results. Liver metastases from colorectal cancer (CRC) have been a hot research topic for decades. As shown by lots of reports in this area, liver resection is an effective method that can improve the therapeutic results (1-4). How about the other malignant tumors? In recent years, increasing reports are mostly positive, while some reports have different point of view (5-9).
CRC, gastric cancer (GC) and breast cancer (BC) refer to the three most prevalent malignancies in China. Along with the emergence of standardized operation techniques and novel drugs, the prognosis of the three tumors has improved to a great extent. In this study, we retrospectively analyzed liver metastases patients of the three cancers in two hospitals (i.e., Qilu Hospital of Shandong University and The First Affiliated Hospital of Wenzhou Medical University) that underwent liver resection. The main purpose is to find out how much of a difference could surgical operation make in liver metastases of the three different malignant tumors.
Methods
Patients
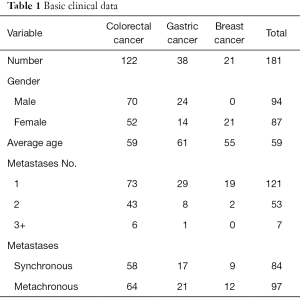
A retrospective analysis on two hospitals was performed to identify all liver metastasis patients with CRC, GC and BC who underwent liver resection between Jan 2002 and Jan 2015. The clinical and follow-up data of patients were obtained from the two hospitals’ medical records. A total of 181 patients, including 122 CRC, 38 GC as well as 21 BC, were involved in the study. The detailed clinical data can be summarized in Table 1.
Full table
Treatment strategy
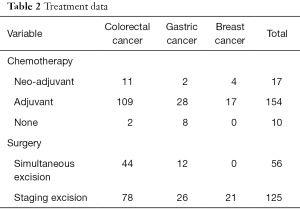
Every patient underwent liver resection on a case-by-case basis. So was the chemotherapy. Neo-adjuvant chemotherapy means the chemotherapy administered prior to liver surgery or primary tumor surgery no more than 8 weeks. Adjuvant chemotherapy means the chemotherapy administered after liver surgery or primary tumor surgery, which includes chemotherapy after primary tumor surgery but prior to liver surgery more than 8 weeks in metachronous liver metastases. Some liver resections may undergo as a homochromous operation with the primary tumor in synchronous metastases, while some liver resections undergo after the primary tumor operation. Though there is still not an international guideline for the surgical resection for liver metastases tumor, the surgical indication for the 181 patients are as follows: the metastases are restricted in liver only and resectable, the primary tumor and the metastases tumor can be R0 resected, and there must be sufficient liver compensation after the resection. The treatment data is concluded in Table 2.
Full table
Statistical analysis
Survival time was calculated from the first liver resection to death or the last contact during follow-up for patients who had liver metastasis resected. Median and actuarial survival were calculated using the Kaplan-Meier method. Meanwhile, relationships of possible prognostic factors to outcomes were calculated using the log-rank methods. When the P value is 0.05 or less than 0.05, we considered it statistical significant, and the analysis was performed using the software of SPSS 16.0. The related survival curves were drawn up by GraphPad Prism5.
Results
Perioperative treatment and survival
In the two hospitals, there were 181 patients who underwent hepatic resections within 13 years. Chemotherapy was utilized for 171 patients including 17 cases of neo-adjuvant chemotherapy. Simultaneous excision was applied in 56 patients including 44 CRC patients as well as 12 GC patients. Staging excision was applied in 125 patients including 78 CRC patients, 26 GC patients and 21 BC patients. Furthermore, all the patients applied R0 resection of liver metastases. No other metastases, such as pulmonary or bone metastases, were detected before the liver operation.
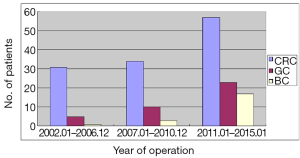
CRC took the biggest proportion of 67.4% (N=122) in all the patients, otherwise proportion is 21.0% (N=38) and 11.6% (N=21) respectively in GC and BC. As shown, the patients had a rising tendency year by year (Figure 1).
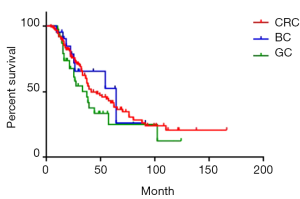
Of all the 181 patients, median survival was 39 months. Moreover, 1-, 3- and 5-year survival rate was 92.3%, 58.3% and 39.2% respectively (Figure 2). Of CRC, GC, and BC liver metastases patients, median survival was 41 months, 37 months and 64 months respectively. Moreover, 1-year survival rate was 90.2%, 86.8%, and 95.2% respectively; 3-year survival rate was 60.0%, 45.9%, and 65.5% respectively; 5-year survival rate was 42.5%, 25.1% and 52.4% respectively. The survival rate of GC group was lower than the other two groups, yet the difference was not significant (P=0.19) (Figure 3).
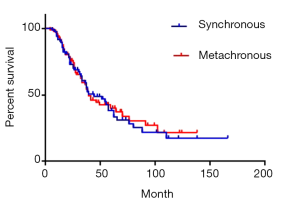
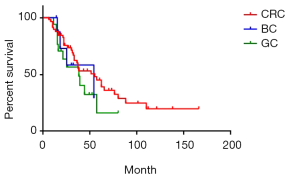
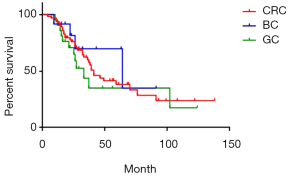
There were 84 cases of synchronous liver metastases patients as well as 97 cases of metachronous liver metastases patients. Median survival was 44 and 39 months respectively. The 1-year survival rate was 90.5% and 91.8% respectively; 3-year survival rate was 59.1% and 57.6% respectively; while 5-year survival rate was 38.3% and 40.1% respectively (P=0.85) (Figure 4). Of CRC, GC, BC synchronous liver metastases patients, median survival was 55, 38 and 54 months respectively; 1-year survival rate was 84.5%, 70.6% and 72.9% respectively; 3-year survival rate was 60.1%, 48.4% and 58.3% respectively; 5-year survival rate was 45.1%, 16.1% and 29.1% respectively (P=0.38) (Figure 5). Of CRC, GC, BC metachronous liver metastases patients, median survival was 41, 33 and 64 months respectively; 1-year survival rate was 82.7%, 76.2% and 91.7% respectively; 3-year survival rate was 64.4%, 43.7% and 69.8% respectively; 5-year survival rate was 38.0%, 34.9% and 69.8% respectively (P=0.47) (Figure 6).
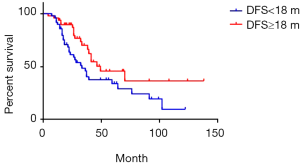
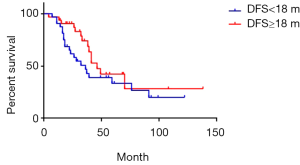
The median DFS was 18 months of 97 cases of metachronous liver metastases patients. Here, DFS means interval between the primary tumor operation and the appearance of liver metastases. We compared the survival of metachronous liver metastases patients whose DFS ≥18 months with those whose DFS <18 months. Median survival of DFS ≥18 and <18 months cases was 49 and 33 months respectively. Additionally, 1-year survival rate was 93.7% and 89.8% respectively; 3-year survival rate was 66.1% and 45.7% respectively; 5-year survival rate was 45.7% and 33.9% respectively (P=0.03) (Figure 7). As for longer DFS, it means longer survival in metachronous liver metastases patients. If DFS related survival was only calculated in CRC patients, the median survival of DFS ≥18 and <18 months cases changed to 46 and 36 months respectively. The 1-year survival rate was 90.5% and 68.4% respectively; 3-year survival rate was 68.7% and 46.7% respectively; 5-year survival rate was 42.3% and 33.4% respectively (P=0.17) (Figure 8). The trend was still that longer DFS leads to longer survival, yet there was no significant difference in statistics. In case of the small sample size, we did not calculate the DFS related survival of GC and BC liver metastases patients.
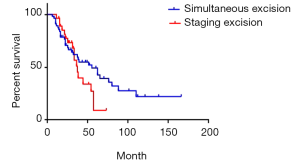
We compared the survival of simultaneous excision cases with staging excision cases of synchronous liver metastases patients. The median survival was 55 and 37 months respectively. The 1-year survival rate was 87.5% and 96.4% respectively; 3-year survival rate was 59.7% and 51.1% respectively; 5-year survival rate was 49.2% and 9.1% respectively (P=0.20) (Figure 9). Apparently, simultaneous excision cases have a lower curve in the beginning as well as obvious higher curve in the end, but sincerely the difference was not significant.
Postoperative mortality, morbidity and recurrence
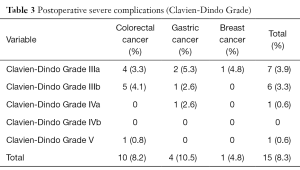
The postoperative severe complications rate of Clavien-Dindo Grade (10) was 8.3% (15 patients) (Table 3). Stromal leak occurred in 6 rectal cancer patients, intestinal obstruction occurred in 2 colon cancer patients and 3 GC patients, while other severe complications include 2 bile leaks and one pulmonary infarction. One 42-year-old female CRC liver metastases patient, with 3 metastases in the right liver, who underwent simultaneous excision of rectal cancer and lobectomy of the right liver by laparoscope, died in 6 months after the operation. A stromal leak happened 10 days after the operation, and ileostomy was made 3 days after the leak. However, severe abdominal infection happened in a week, and then protracted course of hepatapostema, subphrenic abscess and subsequent pulmonary infection attacked the pitiful patients. 6 months after the operation, the patient died of pulmonary infarction and respiratory failure. Other postoperative complications did not give rise to death, and complications were controlled within a month.
Full table
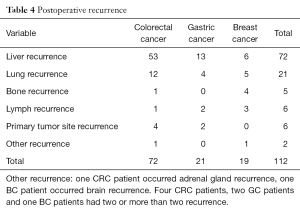
The median time to recurrence after liver resection was 17 months (range, 2–82 months). There were 99 patients (54.7%) and 112 recurrence occurred. The recurrent tumors were identified mostly in the liver and lung, while the other recurrence includes bones, primary tumor site lymph nodes, adrenal gland and brain. Detailed recurrence data can be displayed in the Table 4.
Full table
Discussion
The majority of metastatic liver resection happened to CRC till this day (11). In all reason and emotion experience, most surgeons prefer to liver resection than systemic therapy in resectable colorectal cancer liver metastases (CRLM). Recently, a retrospective case-control study on systemic therapy versus liver resection shows that OS of patients with resectable CRLM (based on CT-scan review) who were treated with systemic therapy was significantly lower than that in case-matched patients who underwent liver resection (12). However, liver resection and systemic therapy has never been in the opposite side, and they supplement each other. An increasing number of CRLM patients had the chance to undergo resection of liver metastases after neoadjuvant chemotherapy (13). Also, newly study shows that liver resection can reduce systemic chemotherapy in return (14). How about the other non-CRLM? Does non-CRLM have the similar behavior with CRLM? In this study, the answer is yes, at least in GC and BC in this study.
In this study, the low morbidity and mortality implied that the operation was safe and feasible. The overall 5-year survival rate of 42.5%, 25.1%, 52.4% and 39.2% respectively in CRC, GC, BC and the cohort indicated favorable outcomes. Indeed, CRCLM, GCLM and BCLM have benefited a lot from liver resection. In multiple studies, 5-year survival was reported over 50% in those CRLM patients with liver resection (15-17). A national study in England shows a mortality with 61.5% in gastric cancer liver metastases (GLM) patients who underwent gastrectomy and hepatectomy (18). Comparison between CRLM and GLM patients who underwent curative hepatectomy revealed that there was no significant difference in the incidence of recurrence and recurrence-free survival, yet the overall survival in CLM was significantly shorter than CRLM (19). Liver resection or other therapeutic methods were more adaptive in resectable breast cancer liver metastases (BLM). Some studies were inclined to surgery (20,21), while some studies did not consider the only way of surgery (8,9,22). A case-control study was carried out to compare outcomes in patients with isolated BLM who underwent surgery and/or ablation with those who underwent conventional medical therapy. As shown, the 5-year OS was 38% and 39% respectively without significant difference (22).
Contrast between different groups reached some consequential conclusion. We concluded that a longer interval between the primary tumor operation and liver metastases means a longer overall survival time after the resection of liver metastases. Otherwise, indicators, such as synchronous liver metastases or metachronous liver metastases, simultaneous excision or staging excision, and different primary tumor did not influence the survival. To keep the data consistent, all positive surgical margin cases were excluded from the study. In cases of the lack of partial data as well as other uncontrollable factors, some significant indicators including CEA, size and number of liver metastases, and primary tumor T & N stage, were not discussed in this study. Liver metastases are not an isolated or upcropping event. One side is the primary tumor, while one side is the condition of liver. Also, the whole-body situation is indispensable. It was reported that inflammatory factors may play a great role in increasing the potential of liver metastases (23,24). Other cytokines like E-cadherin, β-catenin, CD44 and VEGF were very significant in liver metastases as well (25-28). Microenvironments of the liver, especially the neovascularization and capillaries of tumor, deposit (29,30). The survival after liver resection is directly concerned with recurrence and re-metastases, and the mechanism of metastases is especially complex in the aspect of biochemical. Hence, it is really hard to describe the influencing factor of survival from liver metastases by one or few biochemical-related indexes. Otherwise, macroscopic aspect discussion is probably more meaningful.
There were no consensus opinions on the influence of disease free interval between primary tumor and liver metastases. The most extreme example was synchronous metastasis, which might be regarded as zero disease free. Some studies draw a conclusion that synchronous liver metastases from CRC had a worth prognosis after liver resection (31), yet other studies did not mention it’s significance in survival relationship (17,32). The similar thing happened to metachronous liver metastases. Most studies reported a disadvantage of outcome in patients whose disease-free interval was shorter (33-36). According to some experts, this phenomenon might occur by influence of different chemotherapy after primary tumor resection (37). In our study, there was no significant difference between synchronous metastases and metachronous metastases. However, it was significant that a shorter disease free interval led to a shorter survival. The 5-year survival rate was 45.7% and 33.9% respectively in DFS ≥18 and <18 months cases (P=0.03). In terms of this conclusion, it was not to say that shorter DFS patients had no need for operation. Furthermore, we recommend liver resection for those patients only if they are eligible.
Otherwise, there are several inevitable limitations in this retrospective study. To be specific, this study was not pre-established, and not all the patients allowed the same therapeutic standards. Some clinical data were deficient for analysis. For perioperative tumor marker, some clinical data including tumor size were not the same in preoperative imaging materials and pathological descriptions. Additionally, this study had a long-time span of 13 years. During the 13 years, medical technology and treatment idea changed a lot both in surgery therapeutic strategy and systemic therapy. More or less, this might pose some impact on the study. The total number of the cohort was big enough, yet the number of GLM and BLM was relatively small. Hence, we need more clinical data to conduct more significant analysis.
Conclusions
Liver resection is a potential curative treatment in selected metastatic hepatic carcinoma, particularly from CRC, GC and BC. Longer disease free interval between primary tumor resection as well as occurrence of liver metastases indicates longer overall survival after the operation of liver metastases. High recurrence following liver resection of metastases refers to the essential reason of death.
Acknowledgments
We thank Dr. Pan Yuan from Qilu Hospital of Shandong University and Dr. Qingsong Wu from The First Affiliated Hospital of Wenzhou Medical University for offer the convenience in medical record collection.
Funding: This study was supported by a grant from the Zhejiang Provincial Natural Science Foundation of China (No. LY15H160058) and Zhejiang Provincial Medical Technology Science Project (No. 2015KYB245).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.48). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by clinical research ethics board of Wenzhou Medical University and Shandong University (No. 2016-197) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bonney GK, Coldham C, Adam R, et al. Role of neoadjuvant chemotherapy in resectable synchronous colorectal liver metastasis; An international multi-center data analysis using LiverMetSurvey. J Surg Oncol 2015;111:716-24. [Crossref] [PubMed]
- Silberhumer GR, Paty PB, Temple LK, et al. Simultaneous resection for rectal cancer with synchronous liver metastasis is a safe procedure. Am J Surg 2015;209:935-42. [Crossref] [PubMed]
- Krell RW, Reames BN, Hendren S, et al. Surgical Referral for Colorectal Liver Metastases: A Population-Based Survey. Ann Surg Oncol 2015;22:2179-94. [Crossref] [PubMed]
- Macedo FI, Makarawo T. Colorectal hepatic metastasis: Evolving therapies. World J Hepatol 2014;6:453-63. [Crossref] [PubMed]
- Doussot A, Nardin C, Takaki H, et al. Liver resection and ablation for metastatic melanoma: A single center experience. J Surg Oncol 2015;111:962-8. [Crossref] [PubMed]
- Valadares LJ, Costa W Junior, Ribeiro HS, et al. Resection of liver metastasis from neuroendocrine tumors: evaluation of results and prognostic factors. Rev Col Bras Cir 2015;42:25-31. [Crossref] [PubMed]
- Cavanna L, Bodini FC, Stroppa EM, et al. Advanced gastric cancer with liver and lymph node metastases successfully resected after induction chemotherapy with docetaxel, cisplatin and 5-fluorouracil. Chemotherapy 2014;60:224-7. [Crossref] [PubMed]
- Selvakumar D, Dube M, Matey P. Surgical resection of a liver metastasis from breast cancer. Ann R Coll Surg Engl 2015;97:e9-10. [Crossref] [PubMed]
- Lee SY, Sadot E. Does liver resection provide long-term survival benefits for breast cancer patients with liver metastasis? A question yet to be answered. Yonsei Med J 2015;56:309-10. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [Crossref] [PubMed]
- de Ridder JA, van der Stok EP, Mekenkamp LJ, et al. Management of liver metastases in colorectal cancer patients: A retrospective case-control study of systemic therapy versus liver resection. Eur J Cancer 2016;59:13-21. [Crossref] [PubMed]
- Yoo PS, Lopez-Soler RI, Longo WE, et al. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer 2006;6:202-7. [Crossref] [PubMed]
- Malik H, Khan AZ, Berry DP, et al. Liver resection rate following downsizing chemotherapy with cetuximab in metastatic colorectal cancer: UK retrospective observational study. Eur J Surg Oncol 2015;41:499-505. [Crossref] [PubMed]
- Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460-6; discussion 466-7. [Crossref] [PubMed]
- Lordan JT, Riga A, Worthington TR, et al. Early and long-term outcomes of patients undergoing liver resection and diaphragm excision for advanced colorectal liver metastases. Ann R Coll Surg Engl 2009;91:483-8. [Crossref] [PubMed]
- Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759-66. [Crossref] [PubMed]
- Markar SR, Mackenzie H, Mikhail S, et al. Surgical resection of hepatic metastases from gastric cancer: outcomes from national series in England. Gastric Cancer 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Oguro S, Imamura H, Yoshimoto J, et al. Liver metastases from gastric cancer represent systemic disease in comparison with those from colorectal cancer. J Hepatobiliary Pancreat Sci 2016;23:324-32. [Crossref] [PubMed]
- Ruiz A, Wicherts D, Adam R, et al. Resection of liver metastases in breast cancer. Ned Tijdschr Geneeskd 2015;159:A8453. [PubMed]
- Vertriest C, Berardi G, Tomassini F, et al. Resection of single metachronous liver metastases from breast cancer stage I-II yield excellent overall and disease-free survival. Single center experience and review of the literature. Dig Surg 2015;32:52-9. [Crossref] [PubMed]
- Sadot E, Lee SY, Sofocleous CT, et al. Hepatic Resection or Ablation for Isolated Breast Cancer Liver Metastasis: A Case-control Study With Comparison to Medically Treated Patients. Ann Surg 2016;264:147-54. [Crossref] [PubMed]
- Khatib AM, Fallavollita L, Wancewicz EV, et al. Inhibition of hepatic endothelial E-selectin expression by C-raf antisense oligonucleotides blocks colorectal carcinoma liver metastasis. Cancer Res 2002;62:5393-8. [PubMed]
- Auguste P, Fallavollita L, Wang N, et al. The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation. Am J Pathol 2007;170:1781-92. [Crossref] [PubMed]
- Hugo H, Ackland ML, Blick T, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol 2007;213:374-83. [Crossref] [PubMed]
- Brabletz T, Jung A, Reu S, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A 2001;98:10356-61. [Crossref] [PubMed]
- Ouhtit A, Abd Elmageed ZY, Abdraboh ME, et al. In vivo evidence for the role of CD44s in promoting breast cancer metastasis to the liver. Am J Pathol 2007;171:2033-9. [Crossref] [PubMed]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 1997;18:4-25. [Crossref] [PubMed]
- Martin MD, Kremers GJ, Short KW, et al. Rapid extravasation and establishment of breast cancer micrometastases in the liver microenvironment. Mol Cancer Res 2010;8:1319-27. [Crossref] [PubMed]
- Mook OR, Van Marle J, Vreeling-Sindelárová H, et al. Visualization of early events in tumor formation of eGFP-transfected rat colon cancer cells in liver. Hepatology 2003;38:295-304. [Crossref] [PubMed]
- Viganò L, Ferrero A, Lo Tesoriere R, et al. Liver surgery for colorectal metastases: results after 10 years of follow-up. Long-term survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol 2008;15:2458-64. [Crossref] [PubMed]
- Doci R, Gennari L, Bignami P, et al. One hundred patients with hepatic metastases from colorectal cancer treated by resection: analysis of prognostic determinants. Br J Surg 1991;78:797-801. [Crossref] [PubMed]
- Andreou A, Brouquet A, Bharathy KG, et al. Liver resection for liver metastases from nondigestive endocrine cancer: extrahepatic disease burden defines outcome. Surgery 2012;151:851-9. [Crossref] [PubMed]
- Tan MC, Butte JM, Gonen M, et al. Prognostic significance of early recurrence: a conditional survival analysis in patients with resected colorectal liver metastasis. HPB (Oxford) 2013;15:803-13. [Crossref] [PubMed]
- Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 2006;244:524-35. [PubMed]
- Page AJ, Weiss MJ, Pawlik TM. Surgical management of noncolorectal cancer liver metastases. Cancer 2014;120:3111-21. [Crossref] [PubMed]
- Spolverato G, Ejaz A, Azad N, et al. Surgery for colorectal liver metastases: The evolution of determining prognosis. World J Gastrointest Oncol 2013;5:207-21. [Crossref] [PubMed]