
Standard resection procedure can increase the complete resection rate in pediatric patients with locally advanced abdominal neuroblastoma
Introduction
Neuroblastoma (NB) is one of the most common malignant solid tumors in children, accounting for about 8–12% of all childhood malignancies. NB mainly arises from retroperitoneal sympathetic nerve tissue. Due to its insidious onset, the local tumor is often in advanced stage when it is detected. While multidisciplinary treatment mode including chemotherapy, surgery, radiotherapy, and bone marrow transplantation has been applied for the treatment of this disease, surgery remains the most important link. Complete resection (CR) of the primary tumor can dramatically increase the patients’ 5-year survival rate (1). For locally advanced NB (stage III and IV), however, the resection can be difficult because the tumor often surrounds the major blood vessels and organs in the retroperitoneal region. In our center the standard procedure including skeletalization of retroperitoneal vessels and grid-shaped excision of tumor (2) has been applied for the treatment of locally advanced NB, with satisfactory effectiveness.
Methods
Patients
Totally 71 pediatric patients with stage III or IV NB were treated in our center from March 2003 to December 2013. The general data of these patients are shown in Table 1. All the patients received 4–8 cycles of preoperative chemotherapy according to the standard chemotherapy protocol for NB proposed by Children’s Oncology Group, USA. The surgery was performed after the tumor shrank.
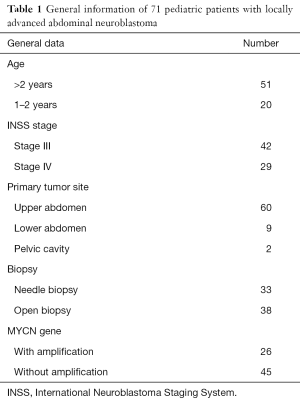
Full table
Surgical methods
The surgical principle was to achieve complete gross resection (CR), whereas near-complete gross resection (NCR) was allowable. Vessels of major organs (e.g., the superior mesenteric vessels and renal vessels) were reconstructed using vascular surgery techniques. Under the premise of ensuring surgical safety and quality of life, some organs such as the body and tail of pancreas and the spleen could be removed.
Under general anesthesia, the patients were placed in a supine position, with their back padded. A transverse abdominal incision closing to the tumor was made; alternately, the biopsy incision was extended. First, the lateral peritoneum of the ascending or descending colon was cut open to turn the colon inwards to expose the anterior side of the tumor. Beginning from the distal side of the tumor, dissection was performed upwards along the inferior vena cava and abdominal aorta, so as to skeletalize these vessels, which was performed along the extravascular space. The perforator branches of tumors and the involved lumbar branches were transected after ligation. In rare cases, the vessels were seriously infiltrated by tumors and thus could not be divided; then, parts of the affected blood vessel walls could be removed first, then the blood vessels could be reconstructed using vascular surgery techniques. The dissection continued along the inferior vena cava to expose the left and right renal veins and along the abdominal aorta to expose the inferior mesenteric artery, left/right renal arteries, superior mesenteric artery, and celiac trunk. With the major vessels as the “grid lines”, the tumor tissues in each “grid” were resected block after block. Notably, the NB following chemotherapy usually had no clear boundaries; thus, the resection scope must be decided according to baseline computed tomographic (CT) findings, so as to ensure the CR of the tumors.
Literature search and statistical analysis
Literature search was performed in PubMed using the search conditions being: command: “neuroblastoma” AND “surgery” AND (“stage 3” OR “stage 4” OR “stage III” OR “stage IV”); fields: “title/abstract”; language: English; and publication date: after the year of 2000.
Statistical analysis was performed using SPSS 16.0 software. Comparison of count data was performed using chi square test, with a P value of less than 0.05 being considered statistically significant.
Results
Clinical outcome
CR or NCR was achieved in 69 of 71 cases (97.2%). Two patients only underwent partial resection. In one of these two patients, the tumor had invaded the head of the pancreas, and the patient's family refused to undergo pancreaticoduodenectomy. In the other patient, the pelvic tumor had invaded the presacral ureter and the affected ureter and parts of the bladder and thus the radical surgery was abandoned. Unilateral nephrectomy was performed in 10 patients because the renal pedicle could not be isolated due to tumor infiltration. Renal vascular reconstruction was performed in 3 cases after parts of renal vein was resected due to tumor infiltration. Superior mesenteric artery was reconstructed after partial resection in 1 case. The body and tail of pancreas and the spleen were removed in 2 cases. Resection of the descending colon was performed in 1 case.
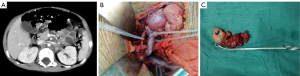
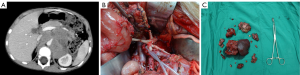
The operative time was 5 h 20 min (2 h 30 min to 8 h 30 min). The intra-operative blood loss was 100–1,200 mL (mean: 550 mL). After the surgery, 7 children suffered from chylous leakage, in whom the drainage tube was withdrawn within 1 month after surgery after adequate drainage, restricted diets, and management for preventing infections; 35 patients developed watery diarrhea, which was improved within 5 postoperative days after symptomatic treatment including fluid replacement and antidiarrheal therapy; 2 patients suffered from incomplete intestinal obstruction during chemotherapy beginning on the 14th postoperative day, and the condition was improved after fasting and fluid replacement. No peri-operative death was noted. No other severe complication such as postoperative bleeding, wound infection, or renal atrophy was detected. Two typical cases are shown in Figures 1,2.
Comparison with literature
Of 95 articles searched from PubMed, 8 randomized controlled trials (RCT) were enrolled for analysis (Table 2). Since watery diarrhea is an inevitable outcome shortly after the CR of retroperitoneal NB, it was not listed as a surgical complication for analysis. The rate of CR/NCR following the standard procedure was higher in our series than those in previous literature (P>0.05), whereas the incidences of complications did not increase (P>0.05).
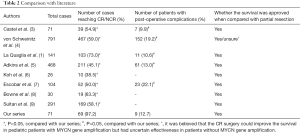
Full table
Discussion
NB seen in patients of different ages has dramatically different response to certain treatment. Infants with NB accompanied by MYCN gene amplification often respond poorly to multidisciplinary treatment including surgery. Castel et al. (10) reported 35 cases of such patients (staged II, III, or IV) and found that the 2-year survival rate was only 30% and the average survival time was only 12 months. In contrast, NB infants without MYCN gene amplification often have much better outcomes. Of 125 pediatric patients reported by De Bernardi et al. (11), the 2-year survival rate was 97.6%, which was not significantly correlated with surgery; thus, they proposed that treatment might not be required for asymptomatic children and a watchful observation might be more feasible. Therefore, infants were not included in our series.
For older (>1 year old) pediatric NB patients, surgical resection still plays an important role, even for advanced NB (stages III and IV). In a retrospective analysis of 69 patients, Bai et al. (12) found that radical resection was the only treatment for patients with non-MYCN-amplified stage III tumours, among whom the 10-year survival reached 84.6%; in patients with MYCN-amplified stage III NB, radical resection plus other multidisciplinary therapies could achieve a 10-year survival of up to 90%. For stage IV NB, Koh et al. (6) believed that radical resection for local tumors were essential and the metastatic lesions could be controlled by chemotherapy; the mean survival of pediatric patients who had achieved local CR was 52.8 months, which was far longer than that (12.2 months) in patients who had only achieved subtotal resection. Bastian et al. (13) also argued that the survival of pediatric patients with stage IV NB was determined by the radical resection of primary tumor and the control of metastatic lesions; a CR surgery not only could increase the local control rate of tumors and decrease complications in the primary sites but also improve the long-term survival. von Schweinitz et al. (4) argued that the radical surgery had no definite role for pediatric NB patient without MYCN gene amplification but did improve the prognosis of those with MYCN gene amplification. However, the locally advanced NB may surround the major organ vessels and thus makes the resection extremely difficult. In Table 2, the highest CR rate was 73% and lowest one was only 38.5%. In our series, preoperative chemotherapy was applied to shrink tumors and reduce intraoperative bleeding, followed by standard surgical operation; as a result, the CR rate reached up to 97.2%. According to our experiences,skeletonization of the major vessels in the retroperitoneal region along the extravascular space, followed by grip-shaped resection of tumors, is the key step in ensuring the safe and CR of the tumors. In rare cases, the vessels were seriously infiltrated by tumors and thus could not be divided; then, the involved organs often need to be resected. In our series, unilateral nephrectomy was performed in 10 patients, resection of the body and tail of pancreas and the spleen in 2 cases, and resection of the descending colon in 1 case, accounting for 18.3% of all cases. Similarly, Adkins et al. (5) reported that the organ resection rate was also 19% in the CR group. In recent years, we have introduced the vascular surgery techniques in the surgical treatment for NB. In our current series, renal vein was reconstructed in 3 cases and superior mesenteric artery was reconstructed in 1 case by vascular grafting; the postoperative organ functions were normal, and no complication was noted. Aminzadeh et al. (14) reported 5 cases who had undergone reconstruction for injured abdominal aorta during surgical resection for stage IV NB and the surgical outcomes were good; they believed that CR surgery could improve the long-term prognosis of patients and thus hgih-risk vascular surgery might be performed for this purpose. In our series, arteries surrounded by tumor tissues were easier to be isolated than veins, along with smaller risk of injuring the abdominal aorta. However, the risk of vascular injury may remarkably increase during a second surgery for recurrence following total or subtotal resection. Thus, adequate preparation for blood transfusion is required before such a surgery; meanwhile, prosthetic vessel should be prepared for dangerous abdominal aorta injury.
In our series, CR was not achieved in two patients, among whom the surgery was abandoned in one patient because the family was not willing to take the risk and the other patient had diffuse tumor invasion into pelvic structures. Cruccetti et al. (15) reported 17 patients with pelvic NB, among whom only 6 had CR; however, the 5-year survival was up to 82%; permanent postoperative neurological complications occurred in 6 patients (35%). These included sciatic nerve palsy, urinary and fecal incontinence, neuropathic bladder, and leg weakness. They proposed that surgical treatment for CR should not be overaggressive for pelvic NB, otherwise it may cause intractable neurological complications. According to our experiences, due to narrow space and rich nerves, CR is difficult to achieve for pelvic NB. In our series, only 1 of 2 patients with pelvic NB achieved CR.
In conclusion, the standard resection procedure can increase the CR rate in pediatric patients with locally advanced abdominal NB without increasing the surgical risk. It is helpful to improve the long-term survival.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.53). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee, and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- La Quaglia MP, Kushner BH, Su W, et al. The impact of gross total resection on local control and survival in high-risk neuroblastoma. J Pediatr Surg 2004;39:412-7; discussion 412-7. [Crossref] [PubMed]
- Bassiri H, Benavides A, Haber M, et al. Translational development of difluoromethylornithine (DFMO) for the treatment of neuroblastoma. Transl Pediatr 2015;4:226-38. [PubMed]
- Castel V, Tovar JA, Costa E, et al. The role of surgery in stage IV neuroblastoma. J Pediatr Surg 2002;37:1574-8. [Crossref] [PubMed]
- von Schweinitz D, Hero B, Berthold F. The impact of surgical radicality on outcome in childhood neuroblastoma. Eur J Pediatr Surg 2002;12:402-9. [Crossref] [PubMed]
- Adkins ES, Sawin R, Gerbing RB, et al. Efficacy of complete resection for high-risk neuroblastoma: a Children's Cancer Group study. J Pediatr Surg 2004;39:931-6. [Crossref] [PubMed]
- Koh CC, Sheu JC, Liang DC, et al. Complete surgical resection plus chemotherapy prolongs survival in children with stage 4 neuroblastoma. Pediatr Surg Int 2005;21:69-72. [Crossref] [PubMed]
- Escobar MA, Grosfeld JL, Powell RL, et al. Long-term outcomes in patients with stage IV neuroblastoma. J Pediatr Surg 2006;41:377-81. [Crossref] [PubMed]
- Browne M, Kletzel M, Cohn SL, et al. Excellent local tumor control regardless of extent of surgical resection after treatment on the Chicago Pilot II protocol for neuroblastoma. J Pediatr Surg 2006;41:271-6. [Crossref] [PubMed]
- Sultan I, Ghandour K, Al-Jumaily U, et al. Local control of the primary tumour in metastatic neuroblastoma. Eur J Cancer 2009;45:1728-32. [Crossref] [PubMed]
- Castel V, Berlanga P. Ototoxicity: a worrying problem for survivors of high-risk neuroblastoma. Transl Cancer Res 2014;3:521-4.
- De Bernardi B, Gerrard M, Boni L, et al. Excellent outcome with reduced treatment for infants with disseminated neuroblastoma without MYCN gene amplification. J Clin Oncol 2009;27:1034-40. [Crossref] [PubMed]
- Bai CQ, Yao YW, Liu CH, et al. Diagnostic and prognostic significance of lysophosphatidic acid in malignant pleural effusions. J Thorac Dis 2014;6:483-90. [PubMed]
- Bastian PJ, Fleischhack G, Zimmermann M, et al. The role of complete surgical resection in stage IV neuroblastoma. World J Urol 2004;22:257-60. [Crossref] [PubMed]
- Aminzadeh S, Vidali S, Sperl W, et al. Energy metabolism in neuroblastoma and Wilms tumor. Transl Pediatr 2015;4:20-32. [PubMed]
- Cruccetti A, Kiely EM, Spitz L, et al. Pelvic neuroblastoma: low mortality and high morbidity. J Pediatr Surg 2000;35:724-8. [Crossref] [PubMed]


