
Apatinib for gastric cancer: are we moving the antiangiogenic strategy any forward?
The promise of angiogenesis in gastric cancer (GC)
GC is a global health burden and it ranks second among the leading cause of cancer related deaths (1). In its advanced phase, this disease is not amenable to cure and the standard approach to metastatic GC patients embraces systemic chemotherapy, which usually includes a combination containing a platinum compound and a fluoropyrimidine (2). Compared to the remarkable improvements reached in the treatment of many other malignancies, the progress we have made in the management of GC—with the notable exception of trastuzumab for HER2 positive disease (3)—seems not that much (4). Indeed, median overall survival (OS) for metastatic patients rarely overcomes 12 months and their 5-year survival rate still remains largely disappointing. In a visionary paper published 45 years ago—much before many of us were born—the potential role of angiogenesis in solid tumors came to the spotlight and set a new treatment paradigm: enchantingly, neovascularisation was crucial for cancer growth and metastasis as it was the only way to guarantee oxygen and nutrients supply to proliferating cancer cells (5). In the same year, President Richard Nixon signed the National Cancer Act into law and historically declared war on cancer. During the following three decades, oncologists enthusiastically believed in the therapeutic implications of this groundbreaking theory and agreed that the chance of targeting angiogenesis would have become the cure-all solution. Because of many thrilling preclinical accomplishments reached in the field, they were convinced that the worldwide cancer plague would have been soon defeated. Unfortunately, they were wrong. Not only cancer has not disappeared and remains the leading cause of death in older adults, but also the healthcare costs to handle it have progressively risen to unprecedented figuresJeny (6). As in many other gastrointestinal tumors, angiogenesis was studied in GC as well (7) and the strategy of blocking vascularization was largely developed. Despite bevacizumab—the pivotal VEGF inhibitor—was tested with great enthusiasm, randomized trials enrolling thousands of GC patients failed to confirm the initial successes of angiogenic inhibition. AVAGAST compared first-line standard chemotherapy (cisplatin and fluoropyrimidine) with either bevacizumab (7.5 mg/kg i.v.) or placebo in 774 patients with locally advanced or metastatic disease (8). Patients whose disease was controlled after six cycles were continued on fluoropyrimidine and bevacizumab until progression, unacceptable toxicity or refusal. Despite tumour response rate (46% vs. 37.4%, OR; P=0.031) and progression-free survival (PFS) (6.7 vs. 5.3 months, HR =0.80; P=0.0037) were both improved, the primary trial endpoint was not met. In fact, the 2-month gain in median OS was not statistically significant in the intention-to-treat population (OS 12.1 vs. 10.1 months; HR =0.87; 95% CI, 0.73–1.03; P=0.1). Similarly, the AVATAR trial showed no survival improvement in Chinese GC patients from the addition to antiangiogenic therapy to any cisplatin and capecitabine-based regimen (HR =1.1) (9). More recently, in the MAGIC-B study 1,100 patients with localized gastric or esophago-gastric adenocarcinoma were randomized to perioperative chemotherapy with or without bevacizumab (10). The addition of the antiangiogenic compound resulted in no benefit at all (HR for OS =1.067; HR for disease-free survival =1.006; response rate 32% for chemotherapy alone vs. 32% for chemotherapy and bevacizumab). Apparently, clinical results of the antiangiogenic strategy applied to GC stand in stark contrast to preclinical experiences. What did go wrong, then? Was first-line a suboptimal disease setting where to test the antiangiogenic strategy? Or rather was bevacizumab the improper drug to test? And, most importantly, after years of strong commitment, have researchers found convincing answers to eventually endorse effective alternatives?
VEGFR-2 and its inhibitors
Even as a more profound understanding of the molecular underpinnings of stomach cancer has markedly advanced the scientific knowledge of the disease, it also lays out the need for new insights, which would help realizing the complex angiogenic process (11). Key among the involved molecules was VEGFR-2, a major signal transducer that regulates endothelial proliferation throughout different pathways. VEGFR-2 structure consists of an extracellular part, a transmembrane portion and an intracellular domain containing two tyrosine-kinase domains and a C-terminal tail (12). The binding between VEGFR-2 and different isoforms of VEGF triggers complex MAP-kinase regulated metabolic cascades, which eventually result in vasodilation, proliferation and cellular migration (13). Unlike bevacizumab, that targets VEGF-A, ramucirumab is a fully human monoclonal IgG directed against VEGFR-2. Ramucirumab was developed both as single agent and in combination with chemotherapy, and it has emerged as a new active therapeutic option for pre-treated patients (14). REGARD was a phase III, placebo-controlled, randomized trial (2:1) comparing ramucirumab to placebo in 355 patients whose disease had progressed during or after a previous chemotherapy treatment (15). The primary endpoint of the study was OS. Results of the trial showed a statistically significant survival improvement for ramucirumab when compared to best supportive care in pretreated GC patients with advanced disease (5.2 vs. 3.8 months; HR =0.776; 95% CI, 0.60–0.99; P=0.047). Moreover, the antiangiogenic drug was very well tolerated with an excellent safety profile and a similar rate of severe toxicity across treatment arms. As expected, patients exposed to ramucirumab had more arterial thrombotic events (2% vs. 0%) and hypertension (8% vs. 3%). RAINBOW was a large, international second-line trial where 655 advanced GC patients were randomized to receive paclitaxel with or without ramucirumab (16). Once again, the primary study endpoint was OS. Median OS was 9.63 months for the combination of paclitaxel and ramucirumab vs. 7.36 months for paclitaxel alone (HR =0.807; 95% CI, 0.678–0.962; P=0.017). Accordingly, RR (28% vs. 16%, P<0.001) and median PFS (4.4 vs. 2.8 months, HR =0.63; 95% CI, 0.53–0.75; P<0.001) were also significantly improved. Based on the results of REGARD and RAINBOW, ramucirumab was the first VEGFR-2 inhibitor approved for clinical use. A randomized first-line phase III trial comparing a standard cisplatin and fluoropyrimidine combination with or without ramucirumab in 616 patients with HER2 negative advanced GC (RAINFALL) has recently completed its accrual. Final results are expected in 2018. Another valuable strategy is to inhibit the intracellular domain of VEGFR-2 exploiting small molecules with TKI activity. Preliminary results of phase I/II clinical trials testing sorafenib, sunitinib, regorafenib, cediranib, orantinib, and MGCD265 have been presented and summarized elsewhere (7).
Apatinib: from preclinical research to clinical practice
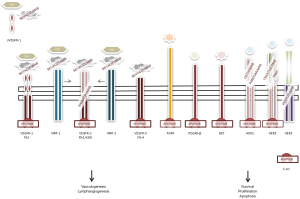
Apatinib (YN968D1) is a novel orally active VEGFR-2 tyrosine kinase inhibitor that blocks its intracellular domain by targeting the ATP-binding site (17), with a binding activity 10-times greater compared to that of other small molecules such as vatalanib or sorafenib (18). Its mechanism of action is shown in Figure 1. Also, apatinib has minor inhibiting activity on platelet-derived growth factor receptor beta, c-kit, and c-src (18). Before its clinical development, the antiangiogenic compound showed to have noteworthy preclinical activity on colorectal (19) and GC cell lines (18). Soon after it was tested in pivotal clinical studies that confirmed its activity (20). Perhaps, the cleanliness in action of this cutting-edge drug may justify its activity and safety profile. In a phase II trial enrolling 144 heavily pretreated GC patients (enrollment criteria included prior lack of response or intolerance to at least two different chemotherapeutic regimens and about one third of them had failed three previous lines of systemic therapy), Li et al. tested two different schedules of apatinib: 850 mg once daily or 425 mg twice daily in a 28-day cycle, since the drug’s plasmatic concentration peaks after approximately 4 h and its mean half-life is about 9 h (21). Primary endpoint of the study was PFS and the comparator arm was placebo. Assuming a drop-off rate of 20%, researchers estimated apatinib to improve median PFS of approximately 2 months. Almost all included patients had a baseline ECOG PS of 1 and 75% of them received previous gastric surgery. Patients exposed to apatinib reported a median PFS that was more than doubled compared to that experienced by patients receiving placebo (median PFS was 3.67, 3.20, and 1.4 months for those receiving apatinib at 850 mg in a unique daily dose, apatinib at 425 mg twice daily, and placebo, respectively). Multivariate Cox regression confirmed the superiority of any apatinib dosing compared to placebo, without significant differences between the two different schedules. Higher response rate (7.3% for those receiving apatinib at 850 mg, 15% for apatinib at 425 mg twice daily, and 0% for placebo) and disease control (24%, 16%, 5%, respectively) were also reported for patients receiving apatinib. The longer PFS translated into an improved median OS, which almost doubled in treated patients and increased from 2.5 months (placebo) to around 4.5 months (apatinib). The antiangiogenic drug was very well tolerated. According to the Li report, toxicity was overall limited, with a favorable safety profile: among patients receiving the fractionated apatinib dose 11% experienced grade 3 hypertension and 13% hand-foot syndrome, which were the leading side effects. Only 4% of patients developed severe proteinuria and none of them eventually had severe renal impairment. Grade 4 toxicities were exceptional. Since the unfractionated 850 mg dosing regimen was equally effective and better tolerated it was recommended for the following phase III trial.
Comment on: “Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients with Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction” by Li J, et al.
This double blind, placebo-controlled, randomized phase III trial was designed in 2010 and undertaken in 32 Chinese centers between 2011 and 2012. After the results of the phase II study were known, aim of the phase III trial was to further confirm the efficacy of the antiangiogenic drug apatinib in patients with advanced GC or gastroesophageal junction adenocarcinoma who had failed previous treatment (22). Among eligibility criteria we would mention disease progression or intolerance to at least 2 previous systemic chemotherapies, measurable disease according to RECIST criteria, ECOG performance status of 0 or 1, and adequate liver, renal and bone marrow function. Importantly, patients older than 70, and those with with inadequate blood pressure control or treated with anticoagulants were not eligible. Patients were randomized 2:1 to receive apatinib (850 mg once daily) or matching placebo, to ensure the treatment to the maximum number of patients. Although one-way crossover at disease progression (placebo to apatinib) had been initially planned, it was eventually not permitted. The study had two co-primary endpoints: OS, defined as the time elapsed from randomization to death for any cause, and PFS, which was described as the time from randomization to disease progression or treatment failure. A complete radiologic assessment was required at baseline, after cycles 2 and 3, and after every 8 weeks thereafter until disease progression. Considering the double primary endpoint, the total type I error was set at 0.05, and appropriately distributed among variables: type I error was allocated at 0.005 for PFS and at 0.02 for OS. A total of 273 patients were randomized, 267 were finally included in the full analysis dataset because 6 of them withdrew the informed consent before starting the assigned treatment. Median age was 58 years in both treatment arms, and male sex was largely prevalent (75%). More PS 0 patients were included in the apatinib arm compared to the placebo arm (27.3% vs. 16.5%). Prior gastrectomy rate was similar across treatment arms (69.3% in the apatinib arm vs. 73.6% in the placebo arm), as it was for sites of metastatic disease >2 (21% vs. 22%), peritoneal involvement (24.4% vs. 27.5%), and previous treatment lines >2 (34.1% vs. 36.3%). Median OS was improved in patients exposed to apatinib (6.5 vs. 4.7 months; HR =0.71; 95% CI, 0.54–0.94; P=0.015). Accordingly, apatinib significantly prolonged median PFS compared to placebo (2.6 vs. 1.8 months; HR =0.44; P<0.001). The preplanned subgroup analysis revealed similar survival improvements regardless the number of metastatic sites, ECOG PS (0 vs. 1), number of previous treatment lines (2 or >2), or age (below or under 65). To put these thought-provoking data into context and define the degree of innovation they bring to the scientific community we should answer three key questions and establish whether these data are true, relevant and, if so, practice-changing.
Are these data true?
The answer is most likely yes. The trial has a strong in-built statistical methodology and a solid internal validity; the same group replicated their previous results obtained in a smaller but similar patients’ population. Nevertheless, several points remain doubtful. Firstly, a concern regarding the trial dosing should be raised. In a multicenter phase II trial of apatinib given at 750 mg per day in pretreated women with triple-negative breast cancer, the vast majority of patients reported grade 3 toxicity and two toxic deaths occurred (23). Although toxicity comparison is hard to set up across different studies and all patients included in the Hu’s study were female, it is unclear why breast cancer patients should have tolerated apatinib differently from GC ones. Even more odd, bearing in mind that GC patients aged over 70 were excluded from the randomized trial. The overall safety profile of apatinib, however, appeared reassuring: the author reported low rates of severe toxicity and the number of patients that required a dose reduction was limited (21%). In particular, grade 3–4 hypertension was noted in 4.5% of patients, significant proteinuria in 2.3%, HFS in 8.5%. These data compare similarly with those of regorafenib, as recently reported (24). In the INTEGRATE trial, 147 patients were randomized 2:1 to receive regorafenib (160 mg daily, days 1–21 on a 28-day cycle) or double-dummy placebo after the failure of 1 or 2 lines of systemic chemotherapy. Safety and toxicity was a secondary trial endpoint. Ten percent of patients had grade 3–5 hypertension, the rate of severe hand-foot skin reaction was 2%, and at least 9% of enrolled patients had significant transaminase increase.
Secondly, the quality of life (QOL) data the Authors provided are debatable. Although the higher rate of compliance for responding to health-related questionnaires in the apatinib arm (34.7% vs. 7.7%) might reflect the treatment effect over placebo on QOL deterioration, the overall return was vastly suboptimal. QOL data from RAINBOW have been recently published (25), reporting over 75% of patients completing the questionnaires at week 20 from randomization.
Thirdly, an imbalance in PS 0 between the two trial arms was clear (absolute difference was almost 11%) which might have impacted on final survival results.
Are these data relevant?
Indeed, a 2-month improvement in heavily pretreated GC patients is what (or more than) we could have expected from an active drug. Actually, a 1.5-month increase in median OS was reported in the REGARD trial with ramucirumab (15), and similar figures are recurrent with the use of second-line chemotherapy with irinotecan or taxanes (26-28). However, let’s put the trial data in the context of the ESMO (29) and the ASCO (30) magnitude of clinical benefit score scales, which are useful to determine the value of any novel treatment, balancing its clinical benefit against its costs. According to the ESMO scale, apatinib would have a rate of 2 out of 4 (form 2a; grade 1 increased by 1 because of favorable safety profile). According to the ASCO conceptual framework to establish value of cancer treatment options, apatinib would sum 42 points out of a maximum of 130 (2×16 points for OS gain, 10 extra points for palliation bonus). And with the widespread use of ramucirumab, will the following use of apatinib—that insists on the same target—give the same results?
Are these data practice-changing?
Honestly, the answer is no. Not so far, at least. Although the drug has been recently approved and licensed by the Chinese Food and Drug Administration, it was tested only in the Asiatic population, or at least in a part of the Asian population, where the patients tend to be younger and fitter (31). Given the potential different behavior of antiangiogenic drugs in different countries, whether same results might be obtained in dissimilar ethnic groups is currently uncertain. Ongoing trials will bring us the final picture in the near future. For example, an ongoing phase IV study will provide safety and efficacy data on more than 2,000 advanced GC patients (32), and additional studies will investigate the role of the compound both in the maintenance phase after standard first-line chemotherapy (33) and in combination with paclitaxel, that may enhance the activity of different antiangiogenic drugs (34). Since the expression of VEGFR-2 may correlate with aggressive disease biology (12), the use of a VEGFR-2 specific inhibitor might be of great value in the clinical practice. With the dawn of the precision medicine era and the availability of novel comprehensive GC classifications (35,36), using antiangiogenic drugs in specific molecular cancer subgroups may be one of the future steps. Finally, the upcoming drug market price also deserves attention, particularly for a drug that has no established predictive factors. We believe that the creation of agreed-upon thresholds and durable investments in comparative effectiveness research will help clinicians improving country-based, sustainable health care systems (37). While a head-to-head trial of apatinib versus ramucirumab in pretreated GC patients is eagerly awaited, health care professionals’ responsibilities in achieving new compelling data and their ability to unite around common values should pave the way towards building any affordable progress in cancer care (38).
Acknowledgments
In memory of our beloved colleague Emiliana Iaiza [1974–2016], who enchanted us all with her bravery of living and her dignity of dying.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Lei Huang (Department of Gastrointestinal Surgery, the First Affiliated Hospital of Anhui Medical University, Hefei, China; German Cancer Research Center (DKFZ), Heidelberg, Germany).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol 2015;33:1760-9. [Crossref] [PubMed]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182-6. [Crossref] [PubMed]
- Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med 2011;364:2060-5. [Crossref] [PubMed]
- Aprile G, Ongaro E, Del Re M, et al. Angiogenic inhibitors in gastric cancers and gastroesophageal junction carcinomas: A critical insight. Crit Rev Oncol Hematol 2015;95:165-78. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Shen L, Li J, Xu J, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer 2015;18:168-76. [Crossref] [PubMed]
- Cunningham D, Smyth E, Stenning S, et al. Peri-operative chemotherapy ± bevacizumab for resectable gastro-oesophageal adenocarcinoma: Results from the UK Medical Research Council randomised ST03 trial (ISRCTN 46020948). Eur J Cancer 2015;51:S400. [Crossref]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669-76. [Crossref] [PubMed]
- Holmes K, Roberts OL, Thomas AM, et al. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal 2007;19:2003-12. [Crossref] [PubMed]
- Hillen F, Griffioen AW. Tumour vascularization: sprouting angiogenesis and beyond. Cancer Metastasis Rev 2007;26:489-502. [Crossref] [PubMed]
- Aprile G, Ferrari L, Cremolini C, et al. Ramucirumab for the treatment of gastric cancers, colorectal adenocarcinomas, and other gastrointestinal malignancies. Expert Rev Clin Pharmacol 2016;9:877-85. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Ding J, Chen X, Gao Z, et al. Metabolism and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor apatinib in humans. Drug Metab Dispos 2013;41:1195-210. [Crossref] [PubMed]
- Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374-80. [Crossref] [PubMed]
- Chen P, Iruela-Arispe L, Lou L, et al. VEGFr inhibitor YN968D1 xenograft dose response studies against human colon cancer Ls174t and HT29. Proc Amer Assoc Cancer Res 2006;47:abst 1764.
- Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 2010;10:529. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219-25. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer 2014;135:1961-9. [Crossref] [PubMed]
- Pavlakis N, Sjoquist KM, Martin AJ, et al. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J Clin Oncol 2016;34:2728-35. [Crossref] [PubMed]
- Al-Batran SE, Van Cutsem E, Oh SC, et al. Quality-of-life and performance status results from the phase III RAINBOW study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated gastric or gastroesophageal junction adenocarcinoma. Ann Oncol 2016;27:673-9. [Crossref] [PubMed]
- Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306-14. [Crossref] [PubMed]
- Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513-8. [Crossref] [PubMed]
- Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78-86. [Crossref] [PubMed]
- Cherny NI, Sullivan R, Dafni U, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol 2015;26:1547-73. [Crossref] [PubMed]
- Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology Statement: A Conceptual Framework to Assess the Value of Cancer Treatment Options. J Clin Oncol 2015;33:2563-77. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
Study of Apatinib Tablets in the Treatment of Advanced or Metastatic Gastric Cancer - Aprile G, Fanotto V, Garattini SK, et al. The concept of maintenance: may we move it to gastric, pancreatic and liver cancers? WCRJ 2016;3:e713
- Roviello G, Roviello F, Polom K, et al. Apatinib in metastatic gastric cancer: can paclitaxel make the difference? Anticancer Drugs 2016;27:809. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449-56. [Crossref] [PubMed]
- Fasola G, Aprile G, Pinto C. Balancing Clinical Progress With Economic Sustainability: In Quest of a Courageous Solution. J Clin Oncol 2015;33:3841-2. [Crossref] [PubMed]
- Obama B. United States Health Care Reform: Progress to Date and Next Steps. JAMA 2016;316:525-32. [Crossref] [PubMed]


