
Does caffeine intake and coffee consumption associate with endometrial cancer among postmenopausal women in America using NHANES 2003–2012?
Introduction
Endometrial cancer is the most common gynecologic cancer in the United States (US). In 2016, it has been estimated that about 60,050 new cases of endometrial cancer will be diagnosed and about 10,470 women will die from it in the US. It is more common in women who are after menopause. Some endometrial cancer may be hardly found by signs and symptoms before reaching an advanced stage, and the 5-year survival rate for women with stage IV endometrial cancer is just around 16% (1). There are several known risk factors for endometrial cancer, such as obesity, insulin resistance, estrogen use after menopause, and decreased plasma sex hormone binding globulin (SHBG) (2-6). Interestingly, cigarette smoking has been reported as a beneficial factor to endometrial cancer by some studies (7,8).
Coffee has been one of the most widely consumed beverages for centuries. In the United States, 59% of Americans saying they drink coffee every day, while 71% saying they drink coffee at least once per week (9). It contains some beneficial phytochemicals such as antioxidants and anti-mutagenic properties (10,11). Some studies found that coffee consumption may reduce the total cancer incidence (12,13) and even lower the risk of all-cause mortality (14,15). Additionally, coffee consumption may have an inverse association with some type of cancers. In Arnlöv’s study, coffee consumption may reduce the risk of kidney cancer by improving insulin sensitivity (16). Cavin and his colleagues found that cafestol and kahweol in coffee may fight against colorectal cancer and may induce excretion of bile acids and neutral sterols into the colon (17). The results from Setiawan’s study suggested that coffee consumption has an inverse association with risk of liver cancer (18). Nagata’s study found that high intake of caffeine may increase sex hormone-binding globulin, and this hormone change may have an inverse association with breast or endometrial cancer risk (19).
In recent years, the connection between coffee consumption and associated risk of endometrial cancer has held great interest. Several epidemiological studies have shown evidence that coffee consumption has an inverse association with endometrial cancer (20-25). Coffee naturally contains numerous compounds, such as caffeine, antioxidants, diterpenes, acrylamide and furan, whose concentrations vary widely by coffee processing and preparation. Among those compounds, caffeine is the major pharmacological active component in coffee. To our knowledge, no study has attempted to measure and control for caffeine intake directly to see whether caffeine is the key cause associated with endometrial cancer.
Therefore, the main objective of this study was to investigate the association of caffeine intake and endometrial cancer risk using the National Health and Nutrition Examination Survey (NHANES).
Methods
Study population
The NHANES, initiated by the Centers for Disease Control and Prevention (CDC) and National Center for Health Statistics (NCHS), is a population-based survey that assesses the health and nutritional status of adults and children in the United States. It is unique for combining interviews and physical examinations. Every cycle year about 5,000 participants are sampled to be representative of the noninstitutionalized US residents, and it over-samples persons 60 and older, African Americans, Asians, and Hispanics to ensure adequate sample size for evaluation of the subgroups (26). For the purpose of this study, 2003–2012 NHANES were used, which combined five data sets of 2-year cycles (2003–2004, 2005–2006, 2007–2008, 2009–2010 and 2011–2012).
This study limited the analysis to postmenopausal female participants aged 20 years and above who answered the reproductive questionnaire and attended medical examination and dietary recall interview. Self-reported menopause was identified when the participants answered “menopause/change of life” to the question, “What is the reason that you have not had a period in the past 12 months?” Endometrial cancer status was identified by questions “Have you ever been told by a doctor or other health professional that you had cancer or a malignance of any kind?” and “What kind of cancer was it?” In this study, a total of 5,847 post-menopausal women aged ≥20 with complete information were included in the analysis, and 84 of them have been diagnosed as endometrial cancer. This analysis was IRB approved by NHANES.
Measurements
Dietary data
The US Department of Agriculture (USDA) developed and validated a multiple-pass dietary recall method for NHANES to collect dietary data. Two dietary recall interviews are conducted in person by trained dietary interviewers. The first one was a face-to-face interview in the Mobile Examination Center (MEC), and the second one would be done in 3–10 days later by telephone. Participants reported an uninterrupted listing of all foods and beverages consumed in a 24-hour period the day before (27,28). The volume and dimensions of the food items consumed were estimated by a standard set of measuring tools. The USDA’s food and Nutrient Database for Dietary Studies was used to code dietary intake data and calculate nutrient intakes. USDA calculated nutrient content accurately for every food and beverage. For example, according to their database, 6 ounces restaurant-prepared brewed espresso contains 377 mg caffeine, 6 ounces restaurant-prepared brewed decaffeinated espresso contains 2 mg caffeine, and 6 ounces milk based hot chocolate contains 4 mg caffeine (29).
Data on caffeine intake (mg/day), coffee consumption (g/day), sugar intake (g/day) and energy intake (kcal/day) were obtained from the total nutrient file, which contains total nutrients from food and beverages during a 24-hour period. According to USDA National Nutrient Database for Standard Reference Release 28 (29), one 6 fluid ounce serving of instant regular coffee, which is prepared only with water, should be 179 g. And it contains about 47 mg caffeine. A report showed the average coffee consumption in US is 2.1 cups per day (9). Therefore, in this study, the variable of coffee consumption was categorized in two groups, which were equal or less than two serving (358 g) per day and greater than two serving (358 g) per day. Equivalently, caffeine intake was classed in two groups: equal or less than two serving (93 mg) per day and greater than two serving (93 mg) per day. Sugar intake and energy intake were kept continuous, and continuous caffeine intake and continuous coffee consumption would still be in analysis.
Demographic data
Age, race, education level, poverty level and marital status during the past 30 days were self-reported during the NHANES interview. NHANES categorized race as Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, non-Hispanic Asian, and non-Hispanic multiracial. In this study, Mexican American, other Hispanic, non-Hispanic Asian and non-Hispanic multiracial were grouped in one as “other”. Education level was classed in four groups: less than high school, high school diploma/GED, some college or AA degree, and college graduate or above. Marital status was categorized in three groups: never married, married/living with partner, and divorced/widowed/separated. The income level was measured by using income-to-poverty ratio: the ratio of family or unrelated individual income to their appropriate poverty threshold, which is family size adjusted. Ratio below 1.00 indicates that the income for the respective family or unrelated individual is below the official definition of poverty.
Reproductive data
Twelve years and older female MEC participants were eligible to answer the reproductive health questionnaire during the NHANES interview. Self-report birth control use history status was identified by question “Ever taken birth control pills?” Information on hormone use history status was obtained from the answer of “Have you ever used female hormones such as estrogen and progesterone?”
Other covariates
Body mass index (BMI) is a measure of body fat based on height and weight. Some studies reported it as related to endometrial cancer. It is calculated as weight in kilograms divided by height in meters squared. In this study, participants were categorized into four BMI groups: underweight/normal (BMI <25), overweight (25≤ BMI <30), obese (30≤ BMI <35), and excessively obese (BMI ≥35).
This study used “pack/year” to measure the smoking status. Average amount of cigarettes the individual participants smoked in 1 year were calculated based on these questions: “Have you smoked at least 100 cigarettes in your entire life?”; “Do you now smoke cigarettes?”; “How old when you first started to smoke cigarettes fairly regularly?”; “How old were you when you last smoked cigarettes?”; “At that time, about how many cigarettes did you usually smoke per day?”; “On how many of the past 30 days did you smoke a cigarette?” and “During the past 30 days, on the days that you smoked, about how many cigarettes did you smoke per day?” After obtaining the total number of cigarettes the participant smoked in 1 year, the “pack/year” was calculated by the total number divided by 20. The participant who smoked less than 100 cigarettes in his/her life was considered as non-smoker.
Statistical analyses
Descriptive statistics of all the characteristics, including demographic characteristic, dietary intake, and reproductive health status, were presented by cancer status (yes/no). The Rao-Scott chi-square test with an adjusted F statistic and t-test were used to evaluate the difference of the means of continuous variables and the difference of the frequency of categorical variables.
A logistic regression model with appropriate weight was used to evaluate whether caffeine intake or coffee consumption was a predictor of endometrial cancer. The outcome variable was endometrial cancer status. Both univariate and multivariable analyses were applied. In order to determine a final model, the stepwise variable selections (with a P value of 0.05 as entry and removal criteria) were performed. The covariates included demographic characteristics (age, race, education, income-to-poverty ratio, and marital status), dietary intake (sugar intake, calorie intake), reproductive characteristics (birth control use status, hormones use status), BMI, and smoking status. These covariates were forced in the multivariate models.
All statistical analyses were computed using survey commands (e.g., PROC SURVEYMEANS, PROC SURVEYREG, PROC SURVEYLOGISTIC) of SAS 9.2 to incorporate sample weights (30) and adjust for clusters and strata of the complex sample design. The 2-year MEC survey weight divided by 5 applied to each participant in this 10-year survey (31). The difference estimates, adjusted odds ratios (ORs), and the corresponding 95% confidence intervals (Cls) of those variables were calculated. All P values were 2-sided and considered as significant when <0.05.
Results
The distribution of all characteristics in women with or without an endometrial cancer history after applying sample weights is shown in Table 1. Among post-menopausal women who self-reported a history of endometrial cancer, they were most often white (white 84.76% vs. black 6.13% vs. 9.12% Hispanic/other), of higher education level (less than high school 22.16% vs. high school diploma/GED 29.94%, some college or AA degree 21.34% vs. college graduate or above 26.55%) and married or living with partner (never married 2.80% vs. married/living with partner 62.28% vs. divorced/widowed/separated 34.91%). Post-menopausal women who had an endometrial cancer history took in less caffeine daily than those who did not have an endometrial cancer history (126.48±15.4977 vs. 166.43±3.7125, respectively. P value =0.0182). Post-menopausal women who drank ≤358 grams (2 cups of 6 oz) coffee daily had a significantly different rate of having an endometrial cancer history (did not drink coffee 31.80% vs. drank ≤358 g coffee daily 39.96% vs. drank >358 g coffee daily 28.24%, P value=0.0250). Post-menopausal excessively obese women (BMI ≥35) had an almost significantly different rate of endometrial cancer history than other groups (underweight/normal 26.49% vs. overweight 19.66% vs. obese 22.13% vs. excess obese 31.72%, P value =0.0528). Post-menopausal women who had a history of birth control use and who did not had one had a significant different rate of holding an endometrial cancer history than the group did not use any birth control (46.88% vs. 53.12%, respectively. P value =0.0132).
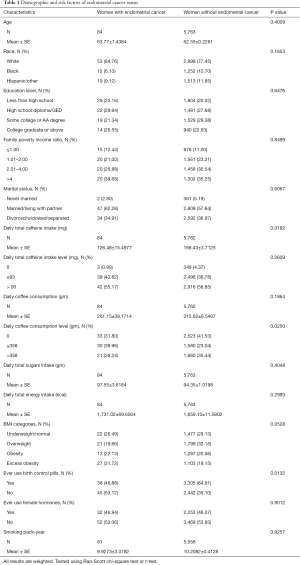
Full table
The univariate and multivariate logistic regression analysis results are shown in Table 2; the appropriate weights and clusters and strata adjustments of the complex sample design are applied. Compared to post-menopausal women who had no caffeine intake, both of two other groups, those who intake ≤93 mg and >93 mg caffeine daily, were 4.134 (95% CI: 1.187–14.397; P value =0.0094) and 5.257 (95% CI: 1.502–18.400; P value =0.0258) times more likely to have endometrial cancer, respectively. Compared to post-menopausal women who did not drink coffee, women who drank ≤358 g of coffee daily had a significant higher rate of developing endometrial cancer (OR =7.650; 95% CI: 2.146–27.264; P value =0.0017). Meanwhile, post-menopausal women who drank >358 g of coffee daily were 3.514 (95% CI: 0.975–12.667; P value =0.0547) times more likely to have endometrial cancer. Black post-menopausal women were significantly less likely to develop endometrial cancer than white post-menopausal women (OR =0.523; 95% CI: 0.274–0.999; P value =0.0496). Compared to post-menopausal women who never use birth control pills, those who had a history of birth control pill use were 0.477 (95% CI: 0.261–0.872; P value =0.0162) times less likely to have endometrial cancer.
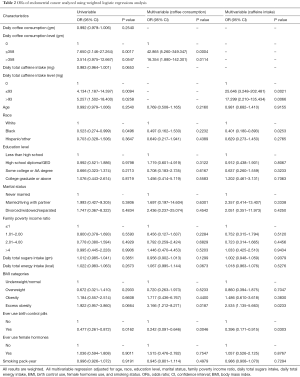
Full table
After controlling for age, race, education level, income-to-poverty ratio, marital status, sugar intake, calorie intake, BMI, birth control use history, female hormones use history, and smoke status, the first multivariate logistic regress model showed that, compared to post-menopausal women who did not intake any caffeine, women who took in ≤93 mg of caffeine in a day were 25.646 (95% CI: 3.248–202.481; P value =0.0021) times more likely to develop endometrial cancer, and women who intake >93 mg of caffeine daily were 17.299 (95% CI: 2.210–135.434; P value =0.0066) times more likely to develop endometrial cancer. In this model, being black and having a history of birth control pill use had significant inverse association with developing endometrial cancer—the ORs were 0.401 (95% CI: 0.180–0.893); P value =0.0253) and 0.396 (95% CI: 0.171–0.915; P value =0.0303, respectively). Being excessively obese increased the rate of endometrial cancer significantly (OR =2.535; 95% CI: 1.135–5.663; P value =0.0233).
In the second multivariate model, post-menopausal women who drank ≤358 g of coffee daily were 42.865 (95% CI: 5.260–349.347; P value =0.0004) times more likely to develop endometrial cancer, compared to those who did not drank coffee. Meanwhile, those who drank >358 g of coffee in a day had 16.354 (95% CI: 1.880–142.301; P value =0.0114) times higher rate of endometrial cancer. The rate of endometrial cancer was significantly lower in those who had a history of birth control pill use than in those who did not (OR =0.242; 95% CI: 0.091–0.646, P value =0.0046). Compared to post-menopausal women who were underweight or normal weight, women who were excessively obese increased rate of developing endometrial cancer significantly (OR =3.166; 95% CI: 1.212–8.271; P value =0.0187). However, there is no significant association between race and endometrial cancer in this model.
Discussion
In this large national population-based study of US post-menopausal women, a statistically significant association between caffeine intake and endometrial cancer was observed. Compared to women who didn’t take in any caffeine, daily intake of ≤93 mg caffeine, which is equivalent to 2 cups of 6 oz instant regular coffee, increased endometrial cancer prevalence by almost 26 times. Although the risk was a little lower in those who take in >93 mg caffeine a day, it still was 17 times higher than the risk among women who did not take in any caffeine. In addition, the results in this study showed coffee consumption was a significant risk factor of endometrial cancer as well. Drinking ≤2 cups of 6 oz instant regular coffee daily was nearly 43 times higher possibilities to get endometrial cancer than post-menopausal women who didn’t drink coffee at all. The risk went down remarkably when women drank >2 cups coffee daily: drinking >2 cups coffee daily was only 16 times more likely to have endometrial cancer than women who did not drink any coffee.
Some previous studies claimed that the risk of endometrial cancer appeared to be reduced by coffee consumption (20-24,32). However, some of them did not show strong evidence in regular population (20,22). Additionally, some of them found the inverse association was only between endometrial cancer and high coffee consumption. Gavrilyuk and colleagues reported endometrial cancer risk decreased in women consuming ≥8 cups/day (21). In Friberg’s (32) and Je’s (24) studies, only ≥4 cups of coffee per day were associated with a lower risk of endometrial cancer. And in both of those two studies, the reference group was women who drank one cup or less. In current NHANES study, there was not a sufficiently valid high coffee consumption record. This may blind us to further evidence. Moreover, the reference group in this study was post-menopausal women who did not drink coffee at all. The risk of endometrial cancer was extraordinarily lower among women who drank >2 cups coffee daily than among those who drank ≤2 cups. This study may obtain the same result as previous studies if the reference group is changed. Overall, there was significant evidence to show that coffee consumption is a risk factor of endometrial cancer compared to non-consumption.
The major difference between this study and previous studies, which made this study unique, is that this study directly investigated the association between caffeine intake and endometrial cancer. The NHANES dataset has recorded individual daily caffeine intake from all the self-reported food and beverage consumption. The estimated amount of caffeine intake is detailed and accurate. The association between caffeine intake and endometrial cancer was consistent with the results between coffee consumption and endometrial cancer. Compared to women who didn’t have any caffeine intake, both of fewer caffeine intake (≤93 mg) or larger caffeine intake (>93 mg) were risk factor of endometrial cancer. And above all, the results also showed that the risk among women who intake more caffeine dropped down to 2/3 risk of fewer intake. It may suggest caffeine is the key component to the association between coffee consumption and endometrial cancer. The previous studies, which tried to inquire into what kind of role caffeine plays, grouped exposure as caffeinated coffee consumption and decaffeinated coffee consumption to investigate this association. Giri and his colleagues found the hazard ratios for women who drank ≥2 cups/day for decaffeinated coffee were 0.67 (95% CI: 0.43–1.06) (22), which means no significant association existed. In Je’s investigation, the inverse association they found among women who consumed ≥2 cups/day vs. <1 cup/month (RR =0.78; 95% CI: 0.57–1.08) was also not convincing (24). Besides caffeine, there were several other compounds in coffee, such as antioxidants, diterpenes, etc. that may impact the association between caffeine intake and endometrial cancer. Also, the impact may be related to the method of brewing or other substances such as sweeteners or creamer. However, in this NHANES study, caffeine intake was measured and calculated accurately. Caffeine intake had a significant association with the risk of endometrial cancer. In the further, etiological evidences needs to be found.
In addition, the result showed that excess obesity (BMI ≥35) was a significant risk factor of endometrial cancer. Friedenreich and her colleagues had the same conclusion (33). Obese women tend to have higher circulating estrogen, hyperinsulinemia, and relatively low levels of SHBG (34,35). Those factors have been shown by many studies to increase the risk of endometrial cancer (4,36,37). Unlike estrogen-only hormone-replacement therapy (HRT), birth control pills are combined estrogen-progestogen medicine. Some studies concluded that combined estrogen-progestogen could decrease risk (38-40). In our study, birth control use was suggested as a beneficial factor of endometrial cancer. This observation is consistent with those studies.
Major strengths of this study include its national population-based design with relatively large sample sizes. NHANES oversampled minorities, thus providing adequate sample size for the stratified analyses with validated dietary interview data. Moreover, the dietary consumption records were obtained through 2 days 24-hour recall interview. The trained interviewer recorded detailed information about foods and beverages that participants reported what they consumed during the 24-hour prior to the interview. Participants used a set of standard measurements to estimate volume or amount of their foods or beverages consumption. This way of recording dietary consumption minimizes the recall bias. In addition, USDA provided professional nutrient guidelines to calculate nutrient intake of all exposure and potential confounders accurately. There are about 30 nutrient guidelines for different coffee types, such as instant coffee prepared with water, breakfast blend brewed coffee, restaurant-prepared brewed espresso, milk-based iced mocha, etc. And caffeine from all foods and beverages consumed, such as coffee cake, coca, or tea, has been included. Each type of coffee has a unique nutrient guideline. It made the findings of this study more powerful and convincing. Furthermore, smoking and coffee consumption are highly correlated and cigarette smoking has been suggested to be associated with lower risk of endometrial cancer (7,8). This study has controlled smoking status to eliminate its impact on the association between coffee consumption and endometrial cancer.
However, the potential contribution of this paper is limited for several reasons. First, because coffee intake, cancer status, and other demographic characteristics used in this study were self-reported, measurement errors are inevitable. Second, the cross-sectional design in the NHANES limits conclusions about the causal relationship of risk factors and coffee consumption. Cancer may impact on life behaviors. To make sure having cancer would not change habit of drinking coffee significantly, coffee consumption and caffeine intake between post-menopausal women who have cancer and do not have cancer was investigated previously. As shown in Table 3, the results suggested that there is no difference between those two groups coffee drinking habit. Third, as NHANES records the cancer status as “uterine cancer” not “endometrial cancer”, the total of endometrial cancer may be overestimated. However, nearly all of uterine cancers are endometrial carcinomas (1), resulting in limited bias in this study. Finally, although all analyses were weighted to account for the complex sampling design applied in NHANES, our results may not represent the whole US population due to the survey limitations.
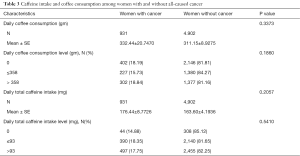
Full table
Conclusions
Despite the limitations, this study uses 10 years’ national data to provide unique and valuable contributions in discussing the association between caffeine intake and endometrial cancer. The results showed that both caffeine intake and coffee consumption is associated with increased risk of endometrial cancer, but risks reduced when having higher intake. Birth control pills use is a factor for reduced risk of endometrial cancer, while excess obesity is significant risk factor.
Acknowledgments
Special acknowledge is given to Tung-Sung Tseng, DrPH. He contributed to the overall study design, data analysis, and interpretation. Sincere gratitude is given to Lu Zhang, MS, and Qingzhao Yu, PhD as well. They provided precious statistical skill support. This study could not be completed without their effort and co-operation. Last but not least, thanks to every friend and responder for the support and willingness to spend some time on this study.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Cancer Research for the series “Social Behavioral and Genetic Risk factors for Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.10.91). The series “Social Behavioral and Genetic Risk factors for Cancer” was commissioned by the editorial office without any funding or sponsorship. TST served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the NHANES ERB.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Endometrial (Uterine) Cancer. Obtained: Aug 4th, 2016. Available online: http://www.cancer.org/acs/groups/cid/documents/webcontent/003097-pdf.pdf.
- Elwood JM, Cole P, Rothman KJ, et al. Epidemiology of endometrial cancer. J Natl Cancer Inst 1977;59:1055-60. [PubMed]
- Kotsopoulos J, Eliassen AH, Missmer SA, et al. Relationship between caffeine intake and plasma sex hormone concentrations in premenopausal and postmenopausal women. Cancer 2009;115:2765-74. [Crossref] [PubMed]
- Lukanova A, Lundin E, Micheli A, et al. Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. Int J Cancer 2004;108:425-32. [Crossref] [PubMed]
- Purdie DM, Green AC. Epidemiology of endometrial cancer. Best Pract Res Clin Obstet Gynaecol 2001;15:341-54. [Crossref] [PubMed]
- Zeleniuch-Jacquotte A, Akhmedkhanov A, Kato I, et al. Postmenopausal endogenous oestrogens and risk of endometrial cancer: results of a prospective study. Br J Cancer 2001;84:975-81. [Crossref] [PubMed]
- Zhou B, Yang L, Sun Q, et al. Cigarette smoking and the risk of endometrial cancer: a meta-analysis. Am J Med 2008;121:501-508.e3. [Crossref] [PubMed]
- Viswanathan AN, Feskanich D, De Vivo I, et al. Smoking and the risk of endometrial cancer: results from the Nurses' Health Study. Int J Cancer 2005;114:996-1001. [Crossref] [PubMed]
- Brown N. Single cups and workplace coffee in the NCA’s 2015 consumer trends report. Obtained: Aug 4th, 2016. Available online: http://dailycoffeenews.com/2015/03/17/single-cups-and-workplace-coffee-in-the-ncas-2015-consumer-trends-report
- Bøhn SK, Blomhoff R, Paur I. Coffee and cancer risk, epidemiological evidence, and molecular mechanisms. Mol Nutr Food Res 2014;58:915-30. [Crossref] [PubMed]
- Gómez-Ruiz JA, Leake DS, Ames JM. In vitro antioxidant activity of coffee compounds and their metabolites. J Agric Food Chem 2007;55:6962-9. [Crossref] [PubMed]
- Löf M, Sandin S, Yin L, et al. Prospective study of coffee consumption and all-cause, cancer, and cardiovascular mortality in Swedish women. Eur J Epidemiol 2015;30:1027-34. [Crossref] [PubMed]
- Yu X, Bao Z, Zou J, et al. Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer 2011;11:96. [Crossref] [PubMed]
- Freedman ND, Park Y, Abnet CC, et al. Association of coffee drinking with total and cause-specific mortality. N Engl J Med 2012;366:1891-904. [Crossref] [PubMed]
- Lopez-Garcia E, van Dam RM, Li TY, et al. The relationship of coffee consumption with mortality. Ann Intern Med 2008;148:904-14. [Crossref] [PubMed]
- Arnlöv J, Vessby B, Risérus U. Coffee consumption and insulin sensitivity. JAMA 2004;291:1199-201. [Crossref] [PubMed]
- Cavin C, Holzhaeuser D, Scharf G, et al. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem Toxicol 2002;40:1155-63. [Crossref] [PubMed]
- Setiawan VW, Wilkens LR, Lu SC, et al. Association of coffee intake with reduced incidence of liver cancer and death from chronic liver disease in the US multiethnic cohort. Gastroenterology 2015;148:118-25; quiz e15.
- Nagata C, Kabuto M, Shimizu H. Association of coffee, green tea, and caffeine intakes with serum concentrations of estradiol and sex hormone-binding globulin in premenopausal Japanese women. Nutr Cancer 1998;30:21-4. [Crossref] [PubMed]
- Bandera EV, Williams-King MG, Sima C, et al. Coffee and tea consumption and endometrial cancer risk in a population-based study in New Jersey. Cancer Causes Control 2010;21:1467-73. [Crossref] [PubMed]
- Gavrilyuk O, Braaten T, Skeie G, et al. High coffee consumption and different brewing methods in relation to postmenopausal endometrial cancer risk in the Norwegian women and cancer study: a population-based prospective study. BMC Womens Health 2014;14:48. [Crossref] [PubMed]
- Giri A, Sturgeon SR, Luisi N, et al. Caffeinated coffee, decaffeinated coffee and endometrial cancer risk: a prospective cohort study among US postmenopausal women. Nutrients 2011;3:937-50. [Crossref] [PubMed]
- Gunter MJ, Schaub JA, Xue X, et al. A prospective investigation of coffee drinking and endometrial cancer incidence. Int J Cancer 2012;131:E530-6. [Crossref] [PubMed]
- Je Y, Hankinson SE, Tworoger SS, et al. A prospective cohort study of coffee consumption and risk of endometrial cancer over a 26-year follow-up. Cancer Epidemiol Biomarkers Prev 2011;20:2487-95. [Crossref] [PubMed]
- Zhou Q, Luo ML, Li H, et al. Coffee consumption and risk of endometrial cancer: a dose-response meta-analysis of prospective cohort studies. Sci Rep 2015;5:13410. [Crossref] [PubMed]
- National Certer for Health Statistics. National Health and Nutrition Examination Survey, 2013-2014 Overview. Obtained: Aug 4th, 2016. Available online: http://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013-14_overview_brochure.pdf
- National Certer for Health Statistics. MEC In-Person Dietary Interviews Procedures Manual. Obtained: Aug 4th, 2016. Available online: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_dietarymec.pdf
- National Certer for Health Statistics. Phone Follow-Up Dietary Interviewer Procedures Manual. Obtained: Aug 4th, 2016. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/PhoneFollowup_0506.pdf
- US Department of Agriculture ARS, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Release 28. updated September 2015. Obtained: Aug 4th, 2016. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/usda-national-nutrient-database-for-standard-reference/
- National Certer for Health Statistics. Task 3b: How to Create an Appropriate Subset of Your Data for NHANES Analyses in SAS Survey Procedures. Obtained: Aug 4th, 2016. Available online: http://www.cdc.gov/nchs/tutorials/NHANES/SurveyDesign/Weighting/Task3b.htm
- National Certer for Health Statistics. Key Concepts About Constructing Weights for Combined NHANES Survey Cycles. Obtained: Aug 4th, 2016. Available online: http://www.cdc.gov/nchs/tutorials/NHANES/SurveyDesign/Weighting/Info2.htm
- Friberg E, Orsini N, Mantzoros CS, et al. Coffee drinking and risk of endometrial cancer--a population-based cohort study. Int J Cancer 2009;125:2413-7. [Crossref] [PubMed]
- Friedenreich C, Cust A, Lahmann PH, et al. Anthropometric factors and risk of endometrial cancer: the European prospective investigation into cancer and nutrition. Cancer Causes Control 2007;18:399-413. [Crossref] [PubMed]
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000;106:473-81. [Crossref] [PubMed]
- Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev 2002;11:1531-43. [PubMed]
- Nestler JE, Powers LP, Matt DW, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab 1991;72:83-9. [Crossref] [PubMed]
- Irwin JC, de las Fuentes L, Dsupin BA, et al. Insulin-like growth factor regulation of human endometrial stromal cell function: coordinate effects on insulin-like growth factor binding protein-1, cell proliferation and prolactin secretion. Regul Pept 1993;48:165-77. [Crossref] [PubMed]
- Persson I, Weiderpass E, Bergkvist L, et al. Risks of breast and endometrial cancer after estrogen and estrogen-progestin replacement. Cancer Causes Control 1999;10:253-60. [Crossref] [PubMed]
- Newcomb PA, Trentham-Dietz A. Patterns of postmenopausal progestin use with estrogen in relation to endometrial cancer (United States). Cancer Causes Control 2003;14:195-201. [Crossref] [PubMed]
- Hill DA, Weiss NS, Beresford SA, et al. Continuous combined hormone replacement therapy and risk of endometrial cancer. Am J Obstet Gynecol 2000;183:1456-61. [Crossref] [PubMed]


