
Protease-activated receptor 1 (PAR1): a promising target for the treatment of glioblastoma?
Malignant astrocytomas constitute a spectrum of clinicopathological entities, from low- to high-grade malignancies. The World Health Organization (WHO) classifies these tumors into four grades according to their histological and anaplastic characteristics. Glioblastoma (GBM) (WHO grade 4) is the most common primary brain tumor in adults and also the most malignant (1). It is distinguished pathologically from lower-grade astrocytomas (grades 2 and 3) by the marked presence of necrosis and/or microvascular hyperplasia (2). GBM is characterized by rapid cell proliferation and marked propensity to invade and damage the surrounding normal brain tissue rendering the complete surgical resection impossible. Despite available treatments, including surgical resection, chemotherapy and radiotherapy, the vast majority of patients exhibit a poor median survival of 15 months following diagnosis (3). Therefore, the development of novel therapeutic approaches to treat GBM remains of critical importance (4).
Increasing evidence has supported the hypothesis that a rare subpopulation of cancer cells sharing stem-cell characteristics within GBM has a potent capacity for tumor propagation (5). These cells are termed tumor precursor cells (TPCs); they demonstrate greater tumorigenic potential compared with matched non-stem tumor cells when xenotransplanted into the brains of immunocompromised rodents (6,7). A2B5 antigen has been recognized as a marker for neural progenitor cells, and explants from A2B5+ tumor cells display typical progenitor morphology that clearly indicates their immature state (8). Nunes and colleagues showed that the majority of A2B5+ multipotential progenitor cells differentiate into oligodendrocytes and a minority of these cells differentiate to neurons (9). A2B5+ cells, but not A2B5− cells isolated from GBM, have neural stem cell-like properties (10). Glioma TPCs have marked self-renewal properties and may generate a number of heterogeneous lineages of cancer cells, which eventually constitute a tumor (11). Therefore, TPCs have been proposed as targets for new therapeutic strategies for the treatment of GBM (12).
Messenger RNA profiling has identified a cohort of genes that distinguish A2B5+ glioma TPCs from A2B5+ normal glial progenitor cells, including the F2R gene that encodes protease-activated receptor 1 (PAR1) (13). PAR1 belongs to a family of four distinct G-protein coupled receptors, which share a unique mechanism of activation that relies on the proteolytic cleavage of their N-terminal ectodomains by specific proteases, including blood coagulation factors. Therefore, PAR1 has long been regarded as a “thrombin receptor”, although it is processed by other proteases. Cleavage of PARs unmasks a new N-terminus, which serves as a tethered ligand that binds to the second extracellular domain of the protein, resulting in a variety of cellular responses that exert important roles in hemostasis, thrombosis and vascular biology (14,15). In addition to their roles in physiological processes, PARs contribute to numerous pathological processes, including tumor biology (16,17). In particular, PAR1 promotes transformation of NIH3T3 cells leading to potent focus-forming activity and loss of anchorage- and serum-dependent growth (18). Several studies have demonstrated a correlation between PAR1 expression/activation and a number of pro-tumor responses, including primary growth, evasion of apoptosis, invasion, metastasis, angiogenesis and epithelial-mesenchymal transition (19-23). A number of studies also demonstrated that PAR1 is overexpressed in GBM (24-26). Indeed, upregulation of PAR1 in gliomas correlates with histological malignancy grade (24,25) and has been shown to be a strong prognostic marker for decreased overall survival (25).
Nearly all GBMs exhibit microscopic intravascular thrombosis within tissue specimens and this event has been documented as an additional distinguishing pathological feature of GBM relative to lower-grade astrocytomas (27). The prothrombotic phenotype of GBM has been largely attributed to the potent procoagulant properties of tumor cells. These properties arise from the increased expression of the clotting initiator protein, tissue factor (TF). TF is highly expressed by GBM cell lines (28-30) and its expression levels in human glioma samples correlate with the grade of malignancy in astrocytomas (31,32). Furthermore, TF expression may impact early stages of gliomagenesis by influencing the dormancy of indolent tumor cells. It is believed that tumor-derived TF allows the formation of a permissive microenvironment containing angiogenic and inflammatory cells. The microenvironment orchestrated by TF expression may drive permanent changes in the phenotype, gene expression profile, DNA copy number, and DNA methylation state of the tumor cells that escape from dormancy (33). In accordance with this hypothesis, some studies have demonstrated an ability of TF inhibitors to decrease GBM growth in rodent models. Ixolaris, a tick-derived protease inhibitor, inhibited the in-vivo tumorigenic potential of U87-MG cells in nude mice. The antitumor effect of ixolaris was associated with downregulation of VEGF and reduced tumor vascularization (34), possibly mediated by decreased thrombin generation in the tumor microenvironment (thus affecting PAR1 activation) and/or by decreased PAR2 signaling in tumor cells (35). In the same line, monoclonal antibodies against TF, including CNTO 859 (which blocks TF-mediated thrombin generation) and 10H10 (which blocks TF-PAR2 signaling), reduce tumor cell invasion in vitro (36) as well as tumor growth in vivo in SCID mice (37).
Some studies previously suggested that the coagulopathy in cancer and the expression of hemostasis-related genes are affected by oncogenic alterations (38,39). For example, overexpression and activating mutations of the epidermal growth factor receptor (EGFR) and loss of tumor suppressor PTEN function drive the upregulation of TF in GBM cells (40). EGFR is a transmembrane tyrosine kinase receptor that plays a central role in cell biology. Ligand binding to the extracellular domain of the receptor leads to functionally active dimers, thereby activating the tyrosine kinase domain. Then autophosphorylation of the receptor occurs on multiple tyrosine residues. This leads to recruitment of a range of adaptor proteins and activates a series of intracellular signaling cascades, such as PI3K-Akt and MAPK pathways, that affect gene transcription, resulting in cancer cell proliferation, reduced apoptosis, invasion and angiogenesis (41). The EGFR gene is amplified in 40% to 50% of GBMs and this event is often accompanied by genetic alterations (42). The most common mutant, EGFRvIII, is formed following the deletion of exons 2 to 7, resulting in a constitutively active receptor that lacks a functional ligand-binding domain (43).
Recent large-scale profiling studies of the genome, epigenome and transcriptome showed the existence of four different subtypes of GBM: proneural, neural, classical and mesenchymal. These subtypes are defined by distinct molecular signatures, as exemplified by the expression alteration of PDGFRA/IDH1, GABRA1, EGFR and NF1, respectively (44). Magnus and colleagues (45) interrogated the largest public GBM gene expression database for levels of transcripts linked to the regulation of the hemostatic system. They observed preferential upregulation of TF (F3) and PAR1 (F2R) in classical GBM tumors expressing high levels of EGFR. The differential expression of hemostatic genes in GBM subtypes may have significant implications for the disease biology, prognosis and therapy. A previous in-vitro study showed that the EGFRvIII-dependent GBM cell transformation is associated with the simultaneous overexpression of TF, PAR1, PAR2 and ectopic synthesis of factor VII (FVII). Moreover, as a result of EGFRvIII-dependent transformation, GBM cells become hypersensitive to TF/PAR-mediated signaling and produce ample angiogenic factors (VEGF and IL-8) upon exposure to coagulation FVIIa and synthetic PAR1- or PAR2-activating peptides (46). EGFR as well as PAR1 were upregulated in glioma TPCs as compared to normal glial progenitor cells, suggesting the importance of the axis EGFR-PAR1 in glioma stem cells (13). Therefore, PAR1 can be an effector of EGFR signaling in the classical subtype of GBM, particularly in the TPCs.
Auvergne and colleagues [2015] have nicely demonstrated that PAR1 is overexpressed in a panel of glioma TPCs derived from primary GBMs (47). Increased PAR1 expression was not restricted to A2B5+ TPCs; it was also identified in CD133+/A2B5− TPCs. In addition, PAR1 gene (F2R) expression was enriched across GBM subtypes relative to normal glial progenitor cells, but most prominently in GBMs of the classical subtype. The authors found a significant correlation between PAR1 expression and EGFR amplification as well as CDKN2A and PTEN deletion, all hallmarks of the classical subtype. PAR1 knockdown induced apoptosis and inhibited proliferation, cell-cycle progression and clonal sphere formation of A2B5+ TPCs. In addition, PAR1 silencing suppressed the in-vivo growth and prolonged survival of mice bearing intracranial glioma TPCs. In-vitro studies employing GBM cell lines demonstrate that PAR1 mediates cell signaling through Akt and MAPK pathways (48,49). It remains to be determined whether these signaling pathways are essential in PAR1-mediated pro-tumoral roles in glioma TPCs.
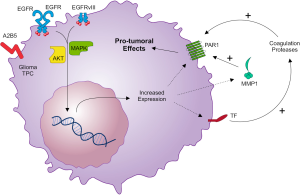
Apart from thrombin, other coagulation enzymes (such as FXa and plasmin) as well as tumor-derived proteases such as matrix metalloprotease-1 (MMP-1), can also activate PAR1 and elicit PAR1 signaling in a thrombin-independent manner (50,51). In gliomas, the upregulation of MMP-1 correlates with progression of histological malignancy grade and poor clinical outcome (25). Interestingly, it has been described that EGFR signaling also contributes to MMP-1 protein upregulation in GBM cells in vitro (52). Expression of PAR1 on vascular endothelium is well documented (15,51,53). The finding of PAR1 activation by MMP-1 suggests that this interaction may also contribute to tumor-host communication in the vascular compartment. Indeed, tumor-derived MMP-1 may induce a prothrombotic, proinflammatory, and adhesive state in endothelial cells expressing functional PAR1 (53). Thus, tumor-endothelial interactions may represent a crucial step in tissue colonization by tumor cells moving through the vascular compartment. Additionally, endothelial cell activation can cause dissociation of cell-cell junctions between endothelial cells as well as cytoskeleton contraction, leading to endothelial barrier disruption (15). Endothelial barrier disruption causes extravasation of plasma carrying coagulation factors (FVII, FX and prothrombin) which in the presence of tumor-derived TF are converted into active proteases, able to cleave PARs in tumor cells (17). Figure 1 summarizes the interactions between EGFR and the TF-PAR1 pathway in glioma TPCs, highlighting possible targets for the treatment of GBM.
Since the 1990s, various pharmaceutical companies have dedicated great effort to searching for potent and selective PAR1 antagonists, particularly in the context of thrombosis and atherosclerosis (51,54-56). Based on the roles of PAR1 in tumor progression, therapeutic strategies towards the inhibition of PAR1 have also been considered. Auvergne and colleagues showed that commercially available PAR1 antagonists, including SCH 530348 (vorapaxar), reduce the in-vitro aggressive properties of glioma TPCs (47). Vorapaxar is approved by the U.S. Food and Drug Administration for the reduction of thrombotic cardiovascular events in patients with a history of myocardial infarction and peripheral artery disease, without a previous stroke or transient ischemic attack (56). Moreover, vorapaxar has been tested in two important clinical trials. Although there were conflicting results concerning the overall advantage of vorapaxar in relation to other cardiovascular drugs, both clinical trials demonstrated increased bleeding risk, including intracranial hemorrhage (56,57). These trials also revealed a number of diplopia (double vision) cases, thus defining an additional relevant side-effect (58). Although the use of vorapaxar may be limited due to its high potential for causing bleeding, it is necessary to investigate efficacy and safety of vorapaxar in the treatment of GBM, since the survival of such patients is very short.
Another innovative strategy for the inhibition of PARs involves using cell-penetrating intracellular antagonists termed pepducins. These compounds are lipopeptides that target the cytoplasmic surface of their cognate receptor thus antagonizing PAR activity in a way that is distinct from classical PAR antagonists (51). PAR1 pepducin P1pal-7 blocked MMP-1-induced PAR1 activation of the Akt survival pathway in breast-cancer cells, resulting in apoptosis in tumor xenografts and inhibition of metastasis to the lungs by up to 88% (59). In principle, it is possible to design pepducins that block PAR-mediated activation of one signaling pathway without affecting other therapeutically desirable signaling pathways. Thus, this class of PAR1 antagonists may have fewer side effects and prove safer. It remains to be determined whether the pepducin compounds will be useful in the clinical setting (51).
Taken together, PAR1 expression in TPCs, along with the establishment of a permissive microenvironment that prompts its activation, may play a major role in GBM progression. A cooperation between EGFR and PAR1 in gliomagenesis might also be considered. Future advances in the treatment of GBM might rely on more effective and better-tolerated therapies, among which PAR1-targeted agents are an attractive option.
Acknowledgments
The authors are grateful to Dr. Martha Sorenson (UFRJ) for the critical reading of the manuscript and to Dr. Rômulo Galvani (romulo.galvani@ufrj.br) for graphic design of the model of glioma TPC.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Ning Huang (Department of Neurosurgery, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.30). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008;359:492-507. [Crossref] [PubMed]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Lima FR, Kahn SA, Soletti RC, et al. Glioblastoma: therapeutic challenges, what lies ahead. Biochim Biophys Acta 2012;1826:338-49.
- Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 2004;64:7011-21. [Crossref] [PubMed]
- Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med 2005;353:811-22. [Crossref] [PubMed]
- Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006;9:391-403. [Crossref] [PubMed]
- Colin C, Baeza N, Tong S, et al. In vitro identification and functional characterization of glial precursor cells in human gliomas. Neuropathol Appl Neurobiol 2006;32:189-202. [Crossref] [PubMed]
- Nunes MC, Roy NS, Keyoung HM, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med 2003;9:439-47. [Crossref] [PubMed]
- Tchoghandjian A, Baeza N, Colin C, et al. A2B5 cells from human glioblastoma have cancer stem cell properties. Brain Pathol 2010;20:211-21. [Crossref] [PubMed]
- Beier D, Hau P, Proescholdt M, et al. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res 2007;67:4010-5. [Crossref] [PubMed]
- Schonberg DL, Lubelski D, Miller TE, et al. Brain tumor stem cells: Molecular characteristics and their impact on therapy. Mol Aspects Med 2014;39:82-101. [Crossref] [PubMed]
- Auvergne RM, Sim FJ, Wang S, et al. Transcriptional differences between normal and glioma-derived glial progenitor cells identify a core set of dysregulated genes. Cell Rep 2013;3:2127-41. [Crossref] [PubMed]
- Gieseler F, Ungefroren H, Settmacher U, et al. Proteinase-activated receptors (PARs) - focus on receptor-receptor-interactions and their physiological and pathophysiological impact. Cell Commun Signal 2013;11:86. [Crossref] [PubMed]
- Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost 2005;3:1800-14. [Crossref] [PubMed]
- Ruf W, Disse J, Carneiro-Lobo TC, et al. Tissue factor and cell signalling in cancer progression and thrombosis. J Thromb Haemost 2011;9:306-15. [Crossref] [PubMed]
- Lima LG, Monteiro RQ. Activation of blood coagulation in cancer: implications for tumour progression. Biosci Rep 2013;33:e00064 [Crossref] [PubMed]
- Martin CB, Mahon GM, Klinger MB, et al. The thrombin receptor, PAR-1, causes transformation by activation of Rho-mediated signaling pathways. Oncogene 2001;20:1953-63. [Crossref] [PubMed]
- Even-Ram S, Uziely B, Cohen P, et al. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med 1998;4:909-14. [Crossref] [PubMed]
- Yang E, Cisowski J, Nguyen N, et al. Dysregulated protease activated receptor 1 (PAR1) promotes metastatic phenotype in breast cancer through HMGA2. Oncogene 2016;35:1529-40. [Crossref] [PubMed]
- Villares GJ, Zigler M, Dobroff AS, et al. Protease activated receptor-1 inhibits the Maspin tumor-suppressor gene to determine the melanoma metastatic phenotype. Proc Natl Acad Sci U S A 2011;108:626-31. [Crossref] [PubMed]
- Kancharla A, Maoz M, Jaber M, et al. PH motifs in PAR1&2 endow breast cancer growth. Nat Commun 2015;6:8853. [Crossref] [PubMed]
- Salah Z, Maoz M, Pokroy E, et al. Protease-activated receptor-1 (hPar1), a survival factor eliciting tumor progression. Mol Cancer Res 2007;5:229-40. [Crossref] [PubMed]
- Carneiro-Lobo TC, Lima MT, Mariano-Oliveira A, et al. Expression of tissue factor signaling pathway elements correlates with the production of vascular endothelial growth factor and interleukin-8 in human astrocytoma patients. Oncol Rep 2014;31:679-86. [PubMed]
- Zhang Y, Zhan H, Xu W, et al. Upregulation of matrix metalloproteinase-1 and proteinase-activated receptor-1 promotes the progression of human gliomas. Pathol Res Pract 2011;207:24-9. [Crossref] [PubMed]
- Kuhn SA, Martin M, Brodhun M, et al. Overexpression of protease-activated receptor type 1 (PAR-1) in glioblastoma multiforme WHO IV cells and blood vessels revealed by NCAM-assisted glioblastoma border labeling. Neurol Res 2014;36:709-21. [Crossref] [PubMed]
- Tehrani M, Friedman TM, Olson JJ, et al. Intravascular thrombosis in central nervous system malignancies: a potential role in astrocytoma progression to glioblastoma. Brain Pathol 2008;18:164-71. [Crossref] [PubMed]
- Ogiichi T, Hirashima Y, Nakamura S, et al. Tissue factor and cancer procoagulant expressed by glioma cells participate in their thrombin-mediated proliferation. J Neurooncol 2000;46:1-9. [Crossref] [PubMed]
- Fernandes RS, Kirszberg C, Rumjanek VM, et al. On the molecular mechanisms for the highly procoagulant pattern of C6 glioma cells. J Thromb Haemost 2006;4:1546-52. [Crossref] [PubMed]
- Monteiro RQ, Lima LG, Gonçalves NP, et al. Hypoxia regulates the expression of tissue factor pathway signaling elements in a rat glioma model. Oncol Lett 2016;12:315-22. [PubMed]
- Guan M, Jin J, Su B, et al. Tissue factor expression and angiogenesis in human glioma. Clin Biochem 2002;35:321-5. [Crossref] [PubMed]
- Jenkins EO, Schiff D, Mackman N, et al. Venous thromboembolism in malignant gliomas. J Thromb Haemost 2010;8:221-7. [Crossref] [PubMed]
- Magnus N, Garnier D, Meehan B, et al. Tissue factor expression provokes escape from tumor dormancy and leads to genomic alterations. Proc Natl Acad Sci U S A 2014;111:3544-9. [Crossref] [PubMed]
- Carneiro-Lobo TC, Konig S, Machado DE, et al. Ixolaris, a tissue factor inhibitor, blocks primary tumor growth and angiogenesis in a glioblastoma model. J Thromb Haemost 2009;7:1855-64. [Crossref] [PubMed]
- Carneiro-Lobo TC, Schaffner F, Disse J, et al. The tick-derived inhibitor Ixolaris prevents tissue factor signaling on tumor cells. J Thromb Haemost 2012;10:1849-58. [Crossref] [PubMed]
- Harter PN, Dützmann S, Drott U, et al. Anti-tissue factor (TF9-10H10) treatment reduces tumor cell invasiveness in a novel migratory glioma model. Neuropathology 2013;33:515-25. [PubMed]
- Magnus N, Meehan B, Garnier D, et al. The contribution of tumor and host tissue factor expression to oncogene-driven gliomagenesis. Biochem Biophys Res Commun 2014;454:262-8. [Crossref] [PubMed]
- Rak J, Yu JL, Luyendyk J, et al. Oncogenes, trousseau syndrome, and cancer-related changes in the coagulome of mice and humans. Cancer Res 2006;66:10643-6. [Crossref] [PubMed]
- Anand M, Brat DJ. Oncogenic regulation of tissue factor and thrombosis in cancer. Thromb Res 2012;129:S46-9. [Crossref] [PubMed]
- Rong Y, Belozerov VE, Tucker-Burden C, et al. Epidermal growth factor receptor and PTEN modulate tissue factor expression in glioblastoma through JunD/activator protein-1 transcriptional activity. Cancer Res 2009;69:2540-9. [Crossref] [PubMed]
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 2006;7:505-16. [Crossref] [PubMed]
- Wong AJ, Bigner SH, Bigner DD, et al. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A 1987;84:6899-903. [Crossref] [PubMed]
- Nishikawa R, Ji XD, Harmon RC, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U S A 1994;91:7727-31. [Crossref] [PubMed]
- Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010;17:98-110. [Crossref] [PubMed]
- Magnus N, Gerges N, Jabado N, et al. Coagulation-related gene expression profile in glioblastoma is defined by molecular disease subtype. J Thromb Haemost 2013;11:1197-200. [Crossref] [PubMed]
- Magnus N, Garnier D, Rak J. Oncogenic epidermal growth factor receptor up-regulates multiple elements of the tissue factor signaling pathway in human glioma cells. Blood 2010;116:815-8. [Crossref] [PubMed]
- Auvergne R, Wu C, Connell A, et al. PAR1 inhibition suppresses the self-renewal and growth of A2B5-defined glioma progenitor cells and their derived gliomas in vivo. Oncogene 2016;35:3817-28. [Crossref] [PubMed]
- Dutra-Oliveira A, Monteiro RQ, Mariano-Oliveira A. Protease-activated receptor-2 (PAR2) mediates VEGF production through the ERK1/2 pathway in human glioblastoma cell lines. Biochem Biophys Res Commun 2012;421:221-7. [Crossref] [PubMed]
- Sayyah J, Bartakova A, Nogal N, et al. The Ras-related protein, Rap1A, mediates thrombin-stimulated, integrin-dependent glioblastoma cell proliferation and tumor growth. J Biol Chem 2014;289:17689-98. [Crossref] [PubMed]
- Boire A, Covic L, Agarwal A, et al. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 2005;120:303-13. [Crossref] [PubMed]
- Ramachandran R, Noorbakhsh F, Defea K, et al. Targeting proteinase-activated receptors: therapeutic potential and challenges. Nat Rev Drug Discov 2012;11:69-86. [Crossref] [PubMed]
- Anand M, Van Meter TE, Fillmore HL. Epidermal growth factor induces matrix metalloproteinase-1 (MMP-1) expression and invasion in glioma cell lines via the MAPK pathway. J Neurooncol 2011;104:679-87. [Crossref] [PubMed]
- Goerge T, Barg A, Schnaeker EM, et al. Tumor-derived matrix metalloproteinase-1 targets endothelial proteinase-activated receptor 1 promoting endothelial cell activation. Cancer Res 2006;66:7766-74. [Crossref] [PubMed]
- Maryanoff BE, Zhang HC, Andrade-Gordon P, et al. Discovery of potent peptide-mimetic antagonists for the human thrombin receptor, protease-activated receptor-1 (PAR-1). Curr Med Chem Cardiovasc Hematol Agents 2003;1:13-36. [Crossref] [PubMed]
- Gurbel PA, Bliden KP, Turner SE, et al. Cell-Penetrating Pepducin Therapy Targeting PAR1 in Subjects With Coronary Artery Disease. Arterioscler Thromb Vasc Biol 2016;36:189-97. [PubMed]
- Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 2012;366:1404-13. [Crossref] [PubMed]
- Wang A. Review of vorapaxar for the prevention of atherothrombotic events. Expert Opin Pharmacother 2015;16:2509-22. [Crossref] [PubMed]
- Serebruany VL, Fortmann SD, Rao SV, et al. Vorapaxar and diplopia: Possible off-target PAR-receptor mismodulation. Thromb Haemost 2016;115:905-10. [Crossref] [PubMed]
- Yang E, Boire A, Agarwal A, et al. Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer Res 2009;69:6223-31. [Crossref] [PubMed]


