
MET-inhibitors meet MET mutations in lung cancer
Lung cancer accounts for a leading cause of cancer mortality worldwide. Patients with non-small cell lung cancer (NSCLC) harboring activating mutations in the epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) fusion genes benefit from treatment with EGFR tyrosine kinase inhibitors (TKIs) and ALK-TKIs, respectively. Recently, NSCLC harboring ROS1 or RET fusion genes were also found to be sensitive to respective TKIs. In addition, mesenchymal-to-epithelial transition (MET) protein overexpression and MET amplification have been shown in NSCLC irrespective of EGFR mutation status (1,2).
MET inhibitors including antibodies and TKIs for NSCLC have been investigated in clinical trials. Although MetMAb (onartuzumab) was most hopeful antibody, a phase III study comparing erlotinib plus onartuzumab with erlotinib alone in MET positive NSCLC by an immunohistochemistry (IHC) assay did not show its efficacy (J Clin Oncol 2014;32:abstr 8000). Furthermore, onartuzumab did not confer any clinical benefit in the MET IHC-positive squamous cell NSCLC when combined paclitaxel plus platinum (3). In case of MET-TKI, the result of a phase III study comparing tivantinib (ARQ 197) plus erlotinib (n=526) with erlotinib alone (n=522) was already published (4). Forty-seven point four percent (211/445) of tumor samples had high MET expression, which was defined if intensity on IHC was >2+ in >50% of tumor cells. Eleven point four percent (54/476) had MET copy number >4 and only four patients had MET amplification with MET to chromosome 7 centromere (MET:CEP7) ratio >2. Although tivantinib plus erlotinib increased progression-free survival (PFS) (median PFS, 3.6 vs. 1.9 months; P<0.001), overall survival (OS) was similar (median OS, 8.5 vs. 7.8 months; P=0.81). OS might be improved in patients with MET IHC high expression (hazard ratio: 0.70; 95% CI: 0.49–1.01) (4). Thus, MET-TKI did not seem to be so beneficial for MET IHC-positive NSCLC as was EGFR-TKIs for EGFR-mutant NSCLC.
Under the concept that aberrant MET signaling can cause cancer, activating point mutations of MET were proved to occur in human renal, hepatocellular, and gastric carcinomas (1,2). MET mutations were also clonally selected for during the metastasis of human head and neck cancers, as their frequency increased from 2% in the primary tumors to 50% (5). In NSCLC specimen, there was an alternative splice variant with the 47-amino acid exon 14 (juxtamembrane domain) missing in-frame from the MET (6). The skipped transcript produces a constitutively active MET that lacks an E3 ubiquitin protein ligase (Cbl) promoting MET degradation (7,8). Exon 14 skipping in MET was found in 4.3%, which was more than a total (3.9%) of ALK (1.3%), ROS1 (1.7%), and RET (0.9%) fusions in 230 lung adenocarcinoma (9). Frequencies of driver oncogene aberrations in 319 Japanese lung adenocarcinoma was 53.0% in EGFR, 3.8% in ALK, 1.9% in RET, 0.9% in ROS1, and 2.8% in skipping of MET exon 14, which was less than ALK fusion alone (3.8% vs. 2.8%) (10). Thus, we need to know clinical and genomic backgrounds with NSCLC harboring MET exon 14 mutation.
Awad et al. described unique clinical, molecular, and pathologic features in 28 (3.0%) patients with MET exon 14 mutations among 933 non-squamous NSCLC patients (7). Genomic deletions occurred in 17 (61%) of the 28 patients with MET exon 14 mutations, ranging in size from a 2-base pair deletion to a 193-base pair deletion, and point mutations occurred in 11 (39%). The median age at diagnosis was 72.5 years, 19 (68%) were women, 10 (36%) were never smokers, their stages I/II/III/IV were 13 (46%)/2 (2%)/4 (14%)/18 (64%), and their histologic subtypes were adenocarcinoma (64%), pleomorphic or sarcomatoid carcinoma with an adenocarcinoma component (14%), poorly differentiated NSCLC not otherwise specified (18%), and adenosquamous carcinoma (4%). Four patients with pleomorphic or sarcomatoid histology and MET exon 14 mutations represented 26.7% of 15 total patients with pulmonary sarcomatoid carcinoma. Liu et al. also reported that MET mutations exon 14 were identified in 8 (22%) of 36 pulmonary sarcomatoid carcinoma (11). The patients with MET exon 14 mutations were older than patients with EGFR- and KRAS-mutant NSCLC, were more likely than those with KRAS mutations to be never-smokers and more likely than those with EGFR mutations to have a history of tobacco use (7). A higher percentage of patients with MET exon 14 mutations had stage I disease compared with those with EGFR or KRAS mutations.
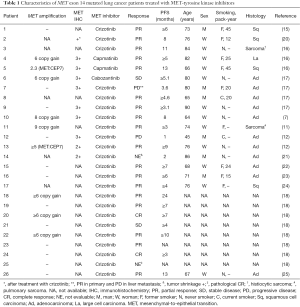
All 28 NSCLC harboring the MET exon 14 in the Awad’ cohort were white, non-Hispanic (7). According to a report from China, MET exon 14 skipping occurred in only 0.9% of lung adenocarcinomas, which was less than half the frequency previously observed in white patients (3%) (12). MET exon 14 mutations occurred at a young median age, 59 years in Chinese patients with stage IV adenocarcinoma, which was similar to the median age of patients with ALK and ROS1 rearrangements. Liu et al. suspected that ethnic difference between Western and Chinese patients could explain the variation. Another report showed that MET exon 14 skipping was detected in 1.3% (23/1,770) of the NSCLC and in 1.6% (21/1,305) of adenocarcinoma in Chinese patients (13). Because MET exon 14 mutation was reported in occurred in 2.8% of Japanese lung adenocarcinomas (10), the difference might be caused by the detection methods. Identifying the intronic mutations responsible for MET exon skipping using genomic DNA is difficult because of their highly diverse locations and the occurrence of passenger mutations (8). In 271 Asian NSCLC (stage I mainly) resected at Korean hospital, 1.8% had exon 14 mutation in MET (14). Although the ethnicity was not described, 19% (10/54) in never-smoking NSCLC patients without EGFR, KRAS, ROS1, BRAF, or ERBB2 (15), 3% (131/4,402) (16), 3% (8/178) (17), 2.8% (205/7,140) (18), and 2.9% (2/70) (19) in lung adenocarcinoma. Thus, overall 1–4% of lung adenocarcinoma may have MET exon 14 mutation, which should be investigated in all NSCLC subtypes including squamous cell, large cell, and sarcomatoid carcinomas (Table 1), especially without other druggable mutations.
Full table
Next-generation sequencing (NGS) also clarified genomic alterations such as KRAS, EGFR, ERBB2, BRAF and TP53 mutations; ALK, ROS1, and RET fusions; MET and MDM2 amplifications in the same specimens (7). Although none of the 28 patients with MET exon 14 mutations had KRAS, EGFR, ERBB2, ALK, ROS1, or RET alterations, mutations of CDKN2A/B, BRAF600E, PIK3CA, PTEN, RB1, ATM, BRCA2, NF1, or ARID2 were co-existed with MET exon 14 mutations. Inactivating mutations in TP53 were observed in 9 patients (32%), and amplification of MDM2, which is a negative regulator of the p53, was observed in 13 patients (46%). When high- and low-level gene copy gains were defined as the MET:CEP7 ratio ≥3 and greater than 1 and less than 3, respectively, 6 (21%) had concurrent high-level MET copy gain and 8 (29%) showed low-level MET copy gain. MET IHC in MET exon 14 mutated NSCLC varied from weak expression to maximum expression. Stage IV NSCLC with MET exon 14 mutation had a significantly higher expression than stage I to III NSCLC with MET exon 14 mutation and than stage IV NSCLC that lacked the mutation. Park et al. showed that MET amplification determined by fluorescent in situ hybridization (FISH) was significantly associated with MET overexpression determined by IHC, however, MET splice mutation was difficult to identify it by IHC or FISH results (19). The importance of concurrent gene mutations, MDM2 or MET amplifications, and MET overexpression remain to be clarified.
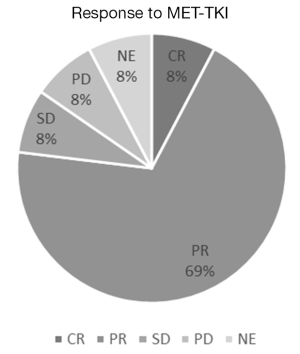
After we have found MET exon 14 mutations in NSCLC with accuracy, we should elucidate whether MET-TKI was effective in such patients or not. The characteristics of MET-TKI-treated NSCLC patients with MET exon 14 mutation were summarized in Table 1, to my knowledge in the literatures. The response to MET-TKIs is shown in Figure 1. Twenty-six patients were treated with MET-TKIs (23 crizotinib, 2 capmatinib; 1 cabozantinib). The responses, complete response (CR)/partial response (PR)/stable disease (SD)/progressive disease (PD)/not evaluable (NE), were observed in 2/18/2/2/2, respectively. One patient, who was evaluated as PD, had PR in primary lesion and PD in liver metastasis (17). Two patients, who were judged as NE (‘unknown’ on Response Evaluation Criteria in Solid Tumors guideline) because tumor response evaluation was not described on the manuscripts, had actually some tumor shrinkage on radiographs. One revealed improvement of the lung mass and a decrease in adrenal metastasis after 5 weeks of crizotinib-treatment, however, drug-induced pneumonitis necessitated crizotinib discontinuation (21). The other was treated with crizotinib as neoadjuvant setting (18). Radiographic response leading to surgical approach was obtained but response was not described. After 2-month treatment with crizotinib, a complete tumor resection and mediastinal lymph node dissection revealed pathological CR. Overall response rate among the 26 patients was 77% (20/26). MET amplification in 21 patients was examined by NGS or FISH and 9 were amplified: CR/PR/SD/PD/NE were observed in 1/7/1/0/0, respectively; response rate was 89% (8/9) in MET amplified NSCLC. In addition, nine tumors examined by MET IHC in samples before MET-TKI’s treatment had all MET overexpression: CR/PR/SD/PD/NE were observed in 0/6/1/1/2, respectively, and response rate was 67% (6/9).
At this time, whether NSCLC patients can benefit from MET-TKIs seems to depend on MET exon 14 mutation irrespective MET overexpression or MET amplification. Recently two cases of crizotinib-sensitive NSCLC harboring high level MET amplification (MET:CEP7 ≥5) without co-incident MET exon 14 mutation, ALK rearrangement, or ROS1 rearrangement were reported (26). Such patients might be investigated in prospective clinical trials using MET-TKI for MET amplified NSCLC such as NCT02544633 trial. Another question is whether central nervous system metastasis is sensitive to MET-TKI similarly to EGFR-TKI or ALK-TKI (27). A MET exon 14 mutated NSCLC patient who had intracranial progression with ongoing response in liver metastases after crizotinib therapy was successfully treated with cabozantinib, which produced rapid intracranial response (28). Prospective trials are needed in order to define the activities of various MET-TKIs for central nervous system metastasis.
Clinical trials of MET-TKIs in NSCLC with MET exon 14 mutations have been conducted. Current studies are available from ClinicalTrials.gov (https://clinicaltrials.gov/ct2/search/index). In the study of ‘Targeted therapy directed by genetic testing in treating patients with advanced refractory solid tumors or lymphomas (NCT02465060; NCI-MATCH)’ is using crizotinib for MET exon 14 mutations. A study of capmatinib (INC280) in NSCLC patients with MET exon 14 alterations who have received prior MET inhibitor is for MET inhibitor-resistant NSCLC (NCT02750215). A phase II study of HMPL-504 (AZD6094, savolitinib) in lung sarcomatoid carcinoma is for MET exon 14 mutation who has failed prior systemic therapy (NCT02897479). A phase II study in lung adenocarcinoma harboring MET exon 14 skipping alterations is using tepotinib (MSC2156119J) (NCT02864992). A phase II study of glesatinib (MGCD265) in patients with NSCLC is for activating genetic alterations in MET (mutation or amplification) (NCT02544633). The studies will clarify whether a variety of MET-TKIs may be useful for NSCLC with MET exon 14 mutations in various situations.
One of the EGFR or ALK-TKI resistant mechanisms is composed of hepatocyte growth factor (HGF)/MET signal activation (29-31). MET activation is induced by binding to its ligand, HGF and mediates cell scatter, growth, proliferation, transformation, and morphogenesis (32,33). MET interacts with several molecules including PI3K and SRC. Thus, excess ligand or bypass signals can abolish the targeted drugs blocking the original oncogene driver and MET signaling is very important in drug resistance. In addition, alterations of MET itself were expected to participate in MET-TKI resistant mechanisms (34,35). Two reports of crizotinib-resistant MET exon 14 mutant NSCLC were described (20,25). An acquired mutation, D1228N in exon 19 of MET, was found at time of progression on crizotinib in a patient with the original exon 14 skipping D1010H mutation (20). Analysis of circulating tumor DNA revealed that Y1230C resistance mutation in MET activation loop occurred in MET D1010H mutant NSCLC post-progression on crizotinib (25). Most MET-TKIs are categorized as three types differing in their mode of binding site in ATP binding pocket in MET kinase. Type I (e.g., crizotinib, capmatinib, tepotinib), type II (e.g., merestinib, cabozantinib, glesatinib) and type III (e.g., MT3) are all ATP competitive inhibitors although tivantinib inhibits ATP binding to the MET kinase in a non-competitive manner (1). Thus, MET-TKI resistant mutations in ATP biding sites (34,35) were expected as T790M in EGFR, and L1196M and G1269A in ALK. MET kinase sites bound to type I MET inhibitors were important interaction sites in Y1230 and D1228 (1,20). Because type II MET inhibitors occupy the ATP binding pocket but also extend into a second pocket that is formed when the side chain of D1222 instead points away from the ATP binding pocket (1), they may be useful for MET secondary mutant NSCLC (20,25). In preclinical tests, a newly developed MET antibody (KTN0073-IgG2), was identified as a potential therapeutic for the treatment of NSCLC with MET exon 14 mutation (36) although it has not been investigated in MET-TKI resistant circumstances. Drugs to overcome MET-TKI resistant NSCLC with MET exon 14 mutation should be developed.
In conclusion, discovery of MET exon 14 mutation in NSCLC similarly to EGFR mutation and ALK fusion was breakthrough because it was targetable oncogenic driver. NSCLC harboring MET exon 14 mutation occupy approximately 1–4% containing adenocarcinoma and other histologic subtypes. Although MET-TKIs have been useful in such a situation, we should wait for the results of ongoing clinical trials for the selected patients. Also, we should clarify the mechanisms of acquired resistance to MET-TKIs which are just beginning to be understood. The therapeutic strategies against drug resistance and intracranial metastasis in NSCLC with MET exon 14 mutation patients should be investigated.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: The author has received honoraria from Pfizer Inc., Japan.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012;12:89-103. [Crossref] [PubMed]
- Kubo T, Yamamoto H, Lockwood WW, et al. MET gene amplification or EGFR mutation activate MET in lung cancers untreated with EGFR tyrosine kinase inhibitors. Int J Cancer 2009;124:1778-84. [Crossref] [PubMed]
- Hirsch FR, Govindan R, Zvirbule Z, et al. Efficacy and Safety Results From a Phase II, Placebo-Controlled Study of Onartuzumab Plus First-Line Platinum-Doublet Chemotherapy for Advanced Squamous Cell Non-Small-Cell Lung Cancer. Clin Lung Cancer 2016. [Epub ahead of print].
- Scagliotti G, von Pawel J, Novello S, et al. Phase III Multinational, Randomized, Double-Blind, Placebo-Controlled Study of Tivantinib (ARQ 197) Plus Erlotinib Versus Erlotinib Alone in Previously Treated Patients With Locally Advanced or Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2667-74. [Crossref] [PubMed]
- Di Renzo MF, Olivero M, Martone T, et al. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene 2000;19:1547-55. [Crossref] [PubMed]
- Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res 2005;65:1479-88. [Crossref] [PubMed]
- Awad MM, Oxnard GR, Jackman DM, et al. MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J Clin Oncol 2016;34:721-30. [Crossref] [PubMed]
- Sunami K, Furuta K, Tsuta K, et al. Multiplex Diagnosis of Oncogenic Fusion and MET Exon Skipping by Molecular Counting Using Formalin-Fixed Paraffin Embedded Lung Adenocarcinoma Tissues. J Thorac Oncol 2016;11:203-12. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. [Crossref] [PubMed]
- Saito M, Shiraishi K, Kunitoh H, et al. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci 2016;107:713-20. [Crossref] [PubMed]
- Liu X, Jia Y, Stoopler MB, et al. Next-Generation Sequencing of Pulmonary Sarcomatoid Carcinoma Reveals High Frequency of Actionable MET Gene Mutations. J Clin Oncol 2016;34:794-802. [Crossref] [PubMed]
- Liu SY, Gou LY, Li AN, et al. The Unique Characteristics of MET Exon 14 Mutation in Chinese Patients with NSCLC. J Thorac Oncol 2016;11:1503-10. [Crossref] [PubMed]
- Zheng D, Wang R, Ye T, et al. MET exon 14 skipping defines a unique molecular class of non-small cell lung cancer. Oncotarget 2016; [Epub ahead of print]. [PubMed]
- Li S, Choi YL, Gong Z, et al. Comprehensive Characterization of Oncogenic Drivers in Asian Lung Adenocarcinoma. J Thorac Oncol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Heist RS, Shim HS, Gingipally S, et al. MET Exon 14 Skipping in Non-Small Cell Lung Cancer. Oncologist 2016;21:481-6. [Crossref] [PubMed]
- Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850-9. [Crossref] [PubMed]
- Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015;5:842-9. [Crossref] [PubMed]
- Schrock AB, Frampton GM, Suh J, et al. Characterization of 298 Patients with Lung Cancer Harboring MET Exon 14 Skipping Alterations. J Thorac Oncol 2016;11:1493-502. [Crossref] [PubMed]
- Park S, Koh J, Kim DW, et al. MET amplification, protein expression, and mutations in pulmonary adenocarcinoma. Lung Cancer 2015;90:381-7. [Crossref] [PubMed]
- Heist RS, Sequist LV, Borger D, et al. Acquired Resistance to Crizotinib in NSCLC with MET Exon 14 Skipping. J Thorac Oncol 2016;11:1242-5. [Crossref] [PubMed]
- Jenkins RW, Oxnard GR, Elkin S, et al. Response to Crizotinib in a Patient With Lung Adenocarcinoma Harboring a MET Splice Site Mutation. Clin Lung Cancer 2015;16:e101-4. [Crossref] [PubMed]
- Jorge SE, Schulman S, Freed JA, et al. Responses to the multitargeted MET/ALK/ROS1 inhibitor crizotinib and co-occurring mutations in lung adenocarcinomas with MET amplification or MET exon 14 skipping mutation. Lung Cancer 2015;90:369-74. [Crossref] [PubMed]
- Waqar SN, Morgensztern D, Sehn J. MET Mutation Associated with Responsiveness to Crizotinib. J Thorac Oncol 2015;10:e29-31. [Crossref] [PubMed]
- Mendenhall MA, Goldman JW. MET-Mutated NSCLC with Major Response to Crizotinib. J Thorac Oncol 2015;10:e33-4. [Crossref] [PubMed]
- Ou SI, Young L, Schrock AB, et al. Emergence of Preexisting MET Y1230C Mutation as a Resistance Mechanism to Crizotinib in NSCLC with MET Exon 14 Skipping. J Thorac Oncol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Caparica R, Yen CT, Coudry R, et al. Responses to crizotinib can occur in high level MET-amplified non-small cell lung cancer independent of MET exon 14 alterations: A brief report. J Thorac Oncol 2016; [Epub ahead of print]. [Crossref]
- Dempke WC, Edvardsen K, Lu S, et al. Brain Metastases in NSCLC - are TKIs Changing the Treatment Strategy? Anticancer Res 2015;35:5797-806. [PubMed]
- Klempner SJ, Borghei A, Hakimian B, et al. Brief Report: Intracranial Activity of Cabozantinib in MET exon14 Positive NSCLC with Brain Metastases. J Thorac Oncol 2016; [Epub ahead of print]. [Crossref]
- Yano S, Nakagawa T. The current state of molecularly targeted drugs targeting HGF/Met. Jpn J Clin Oncol 2014;44:9-12. [Crossref] [PubMed]
- Isozaki H, Ichihara E, Takigawa N, et al. Non-Small Cell Lung Cancer Cells Acquire Resistance to the ALK Inhibitor Alectinib by Activating Alternative Receptor Tyrosine Kinases. Cancer Res 2016;76:1506-16. [Crossref] [PubMed]
- Ochi N, Isozaki H, Takeyama M, et al. Synergistic effect of pacritinib with erlotinib on JAK2-mediated resistance in epidermal gowth factor receptor mutation-positive non-small cell lung Cancer. Exp Cell Res 2016;344:194-200. [Crossref] [PubMed]
- Ponzetto C, Bardelli A, Zhen Z, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 1994;77:261-71. [Crossref] [PubMed]
- Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 2010;11:834-48. [Crossref] [PubMed]
- Qi J, McTigue MA, Rogers A, et al. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res 2011;71:1081-91. [Crossref] [PubMed]
- Tiedt R, Degenkolbe E, Furet P, et al. A drug resistance screen using a selective MET inhibitor reveals a spectrum of mutations that partially overlap with activating mutations found in cancer patients. Cancer Res 2011;71:5255-64. [Crossref] [PubMed]
- Yang Y, Mandiyan S, Robinson BS, et al. Antitumor Properties of an IgG2-Enhanced Next-Generation MET Monoclonal Antibody That Degrades Wild-Type and Mutant MET Receptors. Cancer Res 2016;76:5788-97. [Crossref] [PubMed]


