
Turning the concept of synthetic lethality on its head
One of the most significant discoveries in cancer treatment over the past 15 years has been the advent of poly(ADP-ribose) polymerase (PARP) inhibitors, and their exploitation of the concept of synthetic lethality in the treatment of BRCA1/2 mutant cancers (1,2). In synthetic lethality, the loss of each gene or protein by itself does not affect cell viability, but the loss of both results in cell death. The goal of this approach is to optimise targeted tumor cell death, while sparing normal tissue (the “magic bullet” first proposed by Paul Ehlrich in the late 19th century). PARP inhibitors in BRCA1/2 mutant tumors have been the classical example of this, where the loss of homologous recombination-mediated DNA repair through BRCA1/2 mutation renders cells sensitive to inhibition or loss of PARP1.
However, clinical trials of PARP inhibitors have not resulted in the anticipated dramatic clinical outcomes. Response rates of 41% in BRCA1/2 mutant ovarian cancer were reported in a phase II study, with 7 of 17 patients experiencing a partial response (3). A further stratified phase II study reported a significant improvement in progression-free survival for patients with a germline BRCA1/2 mutation (11.2 vs. 4.3 months) but this did not translate to an overall survival advantage (4). In addition, there were significant toxicities associated with PARP inhibition, including myelosuppression, nausea, fatigue and a 2.2% risk of developing myelodysplastic syndrome/acute myeloid leukemia (MDS/AML) (4,5).
PARP inhibitor resistance
While the availability of PARP inhibitors for patients with ovarian cancer represents a significant improvement in treatment options, the cause of the observed lack of efficacy of PARP inhibitors in the majority of patients remains in question. Various mechanisms of resistance have been proposed, including reversion mutations where BRCA1/2 function is restored by a secondary mutation (which has been reported to occur in up to 28% of ovarian cancers) (6). However, the clinical relevance of this mechanism is not known, with a number of PARP inhibitor resistant patients found to maintain resistance to PARP inhibition in the presence of BRCA1/2 loss (7). The upregulation of P-glycoprotein drug efflux pumps has also been reported to result in PARP inhibitor resistance (8), although again the clinical relevance of this finding is not known, with disappointing results from clinical studies attempting to reverse this mechanism using targeted inhibition of drug efflux pumps in chemotherapy treatments (9). Loss of 53BP1 has also been shown to result in PARP inhibitor resistance, and has been clinically recognized in BRCA1/2 mutation-associated breast cancer (10).
Shifting the paradigm: synthetic viability
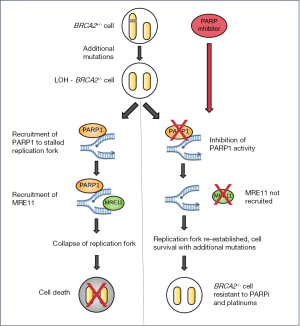
Recently, Sharan and colleagues have challenged the dogma of the synthetic lethality of PARP inhibition in Brca2 mutations and identified a potential novel mechanism for resistance to treatment (11). By exploring the effect of PARP inhibition (using the PARP inhibitor olaparib) and Parp1 knockdown using a conditional Brca2 knockout mouse embryonic stem cell model, they found that loss or inhibition of Parp1 prior to loss of Brca2 function resulted in viable cells lacking functional homologous recombination. Typically, loss of Brca2 would be expected to result in DNA replication fork collapse and cell death. Intriguingly, no synthetic lethality was observed.
These PARPi-resistant and subsequently Brca2-mutant cells (4–8% of those treated) were genomically unstable, with increased sister chromatid exchange and non-homologous end joining. Interestingly, a companion study demonstrated that PARPi resistant tumors were also cross-resistant to topotecan and cisplatin (12). The authors found that loss of Parp1 function restored replication fork stability in the absence of BRCA2 by preventing the recruitment of the nuclease MRE11, an enzyme that is required in the process of DNA replication fork degradation. Continued PARP inhibition was not required for Brca2−/− cell survival, suggesting additional mutational events can promote survival once the cell has survived the initial loss of Brca2 (Figure 1).
Clinical implications
PARP inhibitors are now approved for use in the treatment of BRCA1/2-mutant advanced ovarian cancer, in platinum-sensitive disease in the 4th-line setting in the US (in the 3rd-line setting in Europe). However, an adjuvant trial of PARP inhibitors in early stage BRCA1/2-mutant breast cancer (exploring PARP inhibitor maintenance therapy after adjuvant chemotherapy—OlympiA NCT02032823) is now open. The observed pro-survival effect following PARP inhibition may have important clinical implications for patients receiving PARP inhibitors, particularly as increasing use of PARP inhibitors is being advocated in the adjuvant and prophylactic settings (13). In BRCA2 mutation carriers, exposure to PARP inhibitors, although having a synthetic lethal effect on BRCA2 mutant cancers, may paradoxically accelerate malignancy in “normal” BRCA2 heterozygous cells by promoting survival following spontaneous loss of heterozygosity. Even more worryingly, these cancer cells would be predicted to be resistant to conventional DNA damaging chemotherapeutic agents such as platinums as well as PARP inhibition. Indeed, this may be an important mechanism in considering the increased rate of development of MDS/AML in patients with a BRCA1/2 mutation who receive PARP inhibitor treatment.
Sharan et al.’s study also raises a number of unanswered questions. Is the pro-survival effect of PARP inhibition unique to loss of BRCA2 heterozygosity, or this is a more general phenomenon that also applies to loss of BRCA1 and other genes related to homologous recombination-mediated DNA repair? Is the observed survival of murine Brca2−/− embryonic stem cells with PARP inhibition applicable to loss of heterozygosity in a human epithelial cancer? The authors demonstrated increased epithelial tumor development on a PARP deficient background in Brca2−/− mice, does prolonged exposure to PARP inhibition result in accelerated tumorigenesis in Brca2 heterozygous mice?
Clearly there is an urgent need to better understand the consequences of exposing healthy patients who carry mutations in homologous recombination genes to prolonged PARP inhibition before these agents can be safely used in the prophylactic or adjuvant setting.
Acknowledgments
Funding: EE Parkes was funded by a CR-UK Centre Clinician Fellowship grant; RD Kennedy was funded by the McClay Foundation.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Zheng Li (Department of Gynecologic Oncology, The Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China).
Conflicts of Interest: RD Kennedy receives payment as the Medical director for Almac Diagnostics (UK). EE Parkes has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917-21. [Crossref] [PubMed]
- Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005;434:913-7. [Crossref] [PubMed]
- Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 2011;12:852-61. [Crossref] [PubMed]
- Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014;15:852-61. [Crossref] [PubMed]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244-50. [Crossref] [PubMed]
- Norquist B, Wurz KA, Pennil CC, et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol 2011;29:3008-15. [Crossref] [PubMed]
- Ang JE, Gourley C, Powell CB, et al. Efficacy of chemotherapy in BRCA1/2 mutation carrier ovarian cancer in the setting of PARP inhibitor resistance: a multi-institutional study. Clin Cancer Res 2013;19:5485-93. [Crossref] [PubMed]
- Rottenberg S, Jaspers JE, Kersbergen A, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A 2008;105:17079-84. [Crossref] [PubMed]
- Amiri-Kordestani L, Basseville A, Kurdziel K, et al. Targeting MDR in breast and lung cancer: discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist Updat 2012;15:50-61. [Crossref] [PubMed]
- Jaspers JE, Kersbergen A, Boon U, et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov 2013;3:68-81. [Crossref] [PubMed]
- Ding X, Ray Chaudhuri A, Callen E, et al. Synthetic viability by BRCA2 and PARP1/ARTD1 deficiencies. Nat Commun 2016;7:12425. [Crossref] [PubMed]
- Ray Chaudhuri A, Callen E, Ding X, et al. Replication fork stability confers chemoresistance in BRCA-deficient cells. Nature 2016;535:382-7. [Crossref] [PubMed]
- Vinayak S, Ford JM. PARP Inhibitors for the Treatment and Prevention of Breast Cancer. Curr Breast Cancer Rep 2010;2:190-7. [Crossref] [PubMed]


