
MicroRNAs as novel elements in personalized radiotherapy
Introduction
Radiotherapy (RT) is a widely used cancer treatment modality. Around 50–60% of all cancer patients undergo RT. Despite considerable progress, especially in technical developments, there are still challenges to be met. These include tumor radiation resistance and the killing of normal tissues which result in poor tumor control and/or severe side effects respectively (1,2). Both of these could be counteracted through the administration of agents that enhance tumor radiosensitivity. Ideally, such agents should also reduce the radiosensitivity of healthy tissue. A second major demand to promote and personalize RT in the future is the development of strategies for the prediction of clinical relevant RT parameters, such as tumor control and long-term side effects (3).
A promising class of molecules where targeting may be able to overcome these challenges are the family of microRNAs (miRNAs). These non-protein coding RNA molecules are already showing potential as biomarkers in many clinical situations, and are under development as therapeutics for the treatment of a variety of diseases. Some miRNAs have already reached clinical phase I and II trials (4). In the context of radiation, a wealth of data, mainly obtained from in vitro studies, describes radiosensitizing or radioprotective functions to individual miRNAs that respond during radiation response (5). First reports suggest an application for miRNA signatures as predictive biomarkers in both therapy responses and disease prognosis (see section miRNAs as biomarkers). Most interestingly, beside their established intracellular functions, miRNAs have been detected in body fluids where they may act as signal molecules coordinating cell-cell communication.
A new study describing miR-125b as a putative sensitizer in RT (6) prompted us to discuss the most recent progress in understanding and application of miRNAs for radiosensitivity and RT.
miRNA basics
miRNA are small (~22 nucleotides) non-coding sequences transcribed from the genome under regulatory control. They are non-coding RNA molecules acting as regulators of gene expression (7,8). In general, they negatively regulate gene expression at the post-transcriptional level by preventing efficient translation of messenger RNA (mRNA) or by accelerating mRNA degradation. The miRNA-mediated regulation of cellular phenotype is characterized by a high complexity, because each miRNA is able to act upon several different target genes, whilst one target gene can be regulated by many different miRNAs (9,10). Computional calculations suggest a miRNA-mediated regulation on around 60% of all protein-coding genes. To control such widespread miRNA-mediated functions cells have evolved numerous sophisticated mechanisms to regulate miRNA abundance on the levels of transcription, maturation and stability (11).
miRNA biogenesis and maturation comprises of several steps of processing of a primary nuclear transcript (pri-miRNA) that may be mono- or multicistronic (11). After capping and polyadenylation the pri-miRNA is cleaved by the RNase III enzyme DROSHA into a precursor form (pre-miRNA) and exported into the cytoplasm. The pre-miRNA enters into a complex with a second RNase III enzyme, DICER, where it is processed into the mature form. Subsequently, the 19–23 nucleotide RNA duplex is transfered into the RNA-induced silencing protein complex (RISC). Here the double stranded miRNA is unwound to leave a single stranded miRNA species that is ready to bind a complimentary sequence in the target mRNAs as part of an RISC complex.
Functionally miRNA actions are implicated in the regulation of diverse cellular processes ranging from cellular homeostasis to stress responses (12). Moreover, they have been implicated in many diseases (13).
However, beside their intracellular functions, miRNAs are also present in the extracellular space. miRNAs can be robustly detected in body fluids like plasma, saliva or urine (14). This extracellular miRNA population is heterogeneous and variable. Some miRNAs are packaged into apoptotic bodies, shedding microvesicles, exosomes, or high density lipoprotein (HDL) particles, whereas others are solely complexed with AGO proteins (14). Comparison of miRNA expression profiles in donor cells and in vesicles showed significant differences, suggesting specific packing mechanisms for microvesicle cargo. Moreover the exosomal cargo composition can be altered in response to physiological and pathological conditions such as cellular stress or diseases (15). So far the functions of extracellular miRNAs are not entirely clear, but it was shown that extracellular microvesicle-embedded miRNAs can be transfered and incorporated into recipient cells (16). Most interestingly a recent report described the cell-independent maturation of miRNAs in exosomes from cancer cells (17).
miRNAs as regulators of radiation response
Ionizing radiation (IR) changes intracellular miRNA expression
The essential role of miRNAs in an effective cellular response to radiation exposure was discovered in at least two studies. Kraemer et al. showed in endothelial cells that RNAi downregulation of either of two essential processing enzymes, AGO2 or DICER, leads to a global suppression of miRNA levels, and in turn to an increased level of cell death after γ-irradiation. This indicates a prosurvival function of miRNAs in endothelial cells (18). In accord with this observation Surova et al., found that the miRNA processing enzymes DROSHA and DICER were expressed at higher levels in radioresistant compared to sensitive cell lines (19). Many subsequent studies have shown that IR changes the expression of specific subsets or of individual miRNAs, that have an impact on radiosensitivity (5,20). For miR-21 it was even suggested that the extent of radiation-induced change in the miRNA itself is correlated with high or low cellular radiosensitivity (21). Overall, radiation-induced changes in miRNA expression are transient, dependent upon dose, and are cell type specific. Reports showing common patterns in radiation-triggered deregulation that extend across several cell types, as found for the let-7 family miRNAs or for miR-525-3p, are rare (5,22). For some miRNAs repression in their levels after exposure has been described. Although a mechanistic basis is not yet available reduced levels can be assumed to promote translation of specific miRNA target proteins. Mechanisms proposed to link a radiation response to increased miRNA biogenesis, however, include increased processing of pri-miRNAs through KSPR after phosphorylation by the DNA damage sensor protein ATM or the induction of pri-miRNA transcription by the DNA damage stabilized transcription factor p53 (e.g., miR-34 family) (23,24). Together these findings all hallmark miRNAs to potential key players in determining the response to IR and, by inference, to RT.
miRNAs regulate intracellular radiation response processes
Functionally, miRNAs affect many aspects of tumor radiation sensitivity by regulating cellular key components in cell cycle arrest, DNA damage repair, cell death and radiation related signal transduction (25). On the one hand miRNAs can impair production of proteins essential for DNA damage recognition, signalling, and cell cycle arrest essential to initiate repair. This may lead to lower DNA repair capacity and radiosensitivity. For example miR-24 and miR-451 repress the DNA damage sensor proteins H2AX and ATM (26,27). Additionally, both miRNAs also affect cell cycle progession during stress response. miR-421 targeting of ATM impairs S-phase cell cycle arrest and miR-24 regulates the cell cycle proteins cyclin A and E as well as the retinoblastoma protein.
On the other hand the repair process itself may also be targeted by miRNA action. Examples are miR-210, which represses the RAD52 protein involved in homologous recombination or miR-101, which regulates the DNA-PK kinase essential for non-homologous endjoining (28,29). The subsequent steps in the radiation response, culminating in death or survival may also be influenced by miRNA-mediated regulation of signalling pathways, like PI3K/AKT, nuclear factor-kappa B (NF-κB), mitogen-activated protein kinase (MAPK) and transforming growth factor-beta (TGFβ). Thus, the suppressive effect of miR-221 and miR-222 on the AKT upstream tumor suppressor PTEN leading to increased cell death and radiosensitivity was studied in gastric carcinoma cells (30).
Extrinsic factors, such as local hypoxia and angiogenesis are also important determinants of tumor radiation resistance. Both are critical components that establish the tumor microenvironment and are not surprisingly also regulated by miRNAs. Of particular importance is the hypoxia accelerated miR-210, which enables tumor survival by impairing cell cycle checkpoint activation, homologous recombination and cell death regulation (28,31).
Vesicle-mediated miRNA communication in the radiation response
A completely new field has arisen since the discovery that extracellular miRNAs are potential tools for cell-cell communication during radiation response. In radiation responses of complex tissues the existence of non-targeted effects (bystander or apscopal effects) in cells or tissues that have not been in direct contact with irradiation is well documented. Here again, the underlying mechanisms are not entirely clear (32).
Several recent studies now suggest that microvesicles, especially exosomes and their miRNA cargo, may be able to act as mediators of non-targeted effects. A possible functional link between microvesicles and the radiation response is supported by the finding that p53, upregulated by stabilization during radiation response is also essential for exosome biogenesis (33). In line with this finding Arscott et al. reported a more pronounced increase in radiation-induced exosome release for p53-proficient glioma cells compared to p53-deficient cells (34). Elevated exosome release after irradiation was also reported for human keratinocytes HaCAT (35), breast epithelial tumor cells MCF7 (36), human prostate cell lines (37) and for the head and neck cancer cell line BHY (38). In head and neck as well as glioma cells an increased release of exosomes is accompanied by the increased exosome uptake in irradiated cells. Mechanistically these findings may be explained by a recent study of Hazawa et al., which showed that radiation induces the colocalization of CD29 and CD81 onto the surface of mesenchymal stem cells, where exosomes engage them and are specifically captured (39).
As miRNA changes within microvesicles are reported for other stressors it appears likely that IR will also be able to selectively change the vesicle cargo (40). So far this was only evident in a few studies. One study performed miRNA profiling in exosomes derived from glioblastoma cells and detected a considerable number of up- and downregulated miRNA within the exosomes after irradiation of the donor cells (34). Tang et al. reported increased exosome amounts, together with changes in the expression of four miRNAs in the serum of lung cancer patients after irradiation (41). After in vivo irradiation of rats Beninson et al. showed decreasing miR-142-5p and miR-203 within circulating exosomes, which are associated with immunomodulatory functions (42). Xu et al. showed the participation of exosomal miR-21 in radiation induced bystander effect. He demonstrated that exosomes isolated from irradiated conditioned medium could induce bystander effects by mediating miR-21 transfer from irradiated to bystander cells. Inhibition of miR-21 before irradiation abrogated these effects (43). Nonetheless bystander effects can also be triggered by miRNA transfer without extracellular vesicles. In this context Yuan et al. showed that the extracellular radiation-increased miR-1246 can act as a cell-to-cell messenger which induces radioresistance by targeting the DR5 mRNA in recipient cells independent of extracellular vesicles (44).
miRNAs for clinical applications in RT
miRNAs may have future applications as diagnostic and therapeutic tools in RT. Current data on clinical miRNA applications are mainly reports on circulating or tissue biopsy-localized miRNAs as biomarkers for therapy response and prognosis. Nonetheless, the application of miRNAs as radiosensitizers during RT is being increasingly recognized.
miRNAs as biomarkers
The potential of miRNAs as biomarkers lies in their (I) specificity for the administered treatment; (II) stability in tissues, together with their stable release into body fluids (e.g., blood or urine); (III) fast, robust and economic detection of miRNA expression signatures [even after long-term storage or formalin-fixed parafine embedding (FFPE)] (45).
First studies have reported radiation-induced miRNA changes within tumor tissues which may be used as RT biomarkers. Drebber et al. showed that low intratumoral miR-145 expression post chemo-RT correlated with poor neoadjuvant chemo-RT-response in rectal cancer patients (46). Expression profiling of postoperative RT-resistant and -sensitive non-small cell lung cancer patient samples revealed 12 deregulated miRNAs samples in which the radioresistent cases were correlated with low miR-126 and miR-7a expression (47). Few reports so far suggest the potential use of miRNAs in predicting/quantifying RT-related normal tissue toxicity. Talwar et al. demonstrated in a mouse model that miRNAs can affect mRNA regulation and apoptosis during oral mucositis (48). In another study a potential role of miR-210 in regulating radiation-induced intestinal fibrosis was shown (49).
Accordingly, several reports suggest plasma miRNA expression as novel biomarkers to predict RT response in a clinical context. The analysis of circulating miRNA levels in blood samples of breast cancer patients before and after RT demonstrated that miR-21 and miR-206 were up-regulated while miR-7, -34a, -29, -15 and -16 were down-regulated in radioresistant patients (50). A study in esophageal squamous cell carcinoma patients identified that the plasma miR-16 expression level has considerable clinical value in predicting RT outcomes (51). In line with these findings Summerer et al. identified patient-specific and therapy-responsive miRNAs in the plasma of head and neck cancer patients. High expression of miR-142-3p, miR-186-5p, miR-195-5p, miR-374b-5p and miR-574-3p correlated with poor prognosis (52,53).
Therapeutic applications of miRNAs
miRNAs may also be used as radiosensitizers in RT in the future. In vitro studies suggest a number of candidate miRNAs whose manipulation leads to radiosenzitation. Recently it has been recognized that therapeutic inhibition of miRNAs or mimicking of miRNAs may be a useful tool for overcoming radioresistance during therapy. The usage of miRNAs in therapeutics is attractive as the miRNA effect is tuning of target expression instead of knocking it out which should be less detrimental to healthy tissues. Moreover, as miRNAs can simultaneously target many genes within a pathway or even multiple pathways therapy resistance may be reduced compared to radiosensitizers developed against one target.
New technologies for the silencing of miRNA functions like antimiRs, locked nucleic acid (LNA) antisense oligonucleotides and miRNA sponges, which act as competitors for miRNA-target interactions are now ready to test their application in vivo. Examples are two recent studies showing the treatment of hepatitis C virus infections. One report showed the treatment of chronically infected chimpanzees with a LNA-modified oligonucleotide complementary to miR-122 which leads to the suppression of hepatitis C virus viremia (54). Another study demonstrated an antimiR treatment for chronic hepatitis C viral infection in humans in a phase II clinical trial (55). Moreover, there are also approaches to mimic or overexpress miRNAs, for example by using lipid-formulated mimics and adeno-associated virus vectors. One group treated lung cancer xenografts using liposomal nanoparticles loaded with miR-200c mimics, and showed that this sensitized tumours to irradiation by regulating the cell oxidative stress response (56).
To facilitate miRNA applications in RT systems the selective delivery to target cells in effective concentrations is necessary. A new approach to overcome this issue is the usage of engineered viral particles for cell transduction with miRNA overexpressing or inhibiting vector constructs (57).
Challenges for miRNA applications in RT
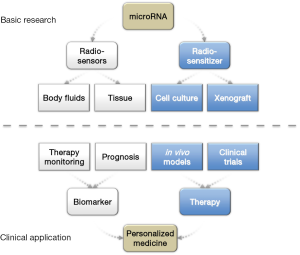
Available data have demonstrated that miRNA expression is a key component in cellular radiosensitivity and Figure 1 summarizes how basic research and clinical miRNA applications may contribute to personalized medicine in future. Early data suggest that it may be possible to use tissue-derived and circulating miRNAs to predict patient response to RT. However further validations, especially prospective trials, are required to establish the usefulness of miRNAs as biomarkers in RT. For example although quantification techniques have high sensitivity and specificity no consensus has been reached regarding best normalization protocols, standardized sample preparation or choice of body fluid (e.g., plasma/serum). Additionally, the small numbers of samples studied may hide bias due to individual sensitivities, concurrent chemotherapies and even assay protocols at all demand independent validation studies.
Current data also challenge the applicability of miRNA targeting for radiosenzitation. With few exceptions, even strong changes in the miRNA content by overexpression or miRNA inhibition only induce small changes in radiation sensitivity. This may be consistent with their biological function as fine-tuners of expression; however, it remains to be investigated if such limited effects are sufficient for putative clinical applications. Regarding limited effect sizes the investigation of cooperative effects resulting from the simultaneous manipulation of several miRNAs may be helpful. Moreover, there is insufficient knowledge about miRNA functions in more clinically relevant experimental scenarios such as dose fractionation/hyperfractionation, use of different radiation qualities (protons, carbon ions) or prevailing hypoxic conditions. Most importantly there is a lack of knowledge about the transferability of in vitro or xenograft data of candidates into in vivo models or patient materials.
Conclusions
In conclusion, research performed thus far supports a relevant role for miRNAs in the future of radiation oncology, which may provide the basis to predict response of RT patients and to develop miRNA-based customized treatments to enhance radiosensitivity. Early studies showed that the usage of miRNAs as biomarker for therapy monitoring and prognosis and therefore for more precise and personalized treatment of patients, is possible. Treatment applications for miRNAs as radiosensitizer are currently restricted to cell culture or xenograft model systems and need to be transferred into in vivo in the future.
Currently least understood is the function of extracellular miRNAs. A detailed investigation of radiation triggered mechanisms for secretion, transfer and function in recipient cells may contribute to the understanding of important RT issues such as apscopal effects or radiation induced secondary tumors.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Hongcheng Zhu (Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.37). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim JH, Jenrow KA, Brown SL. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat Oncol J 2014;32:103-15. [Crossref] [PubMed]
- Willers H, Azzoli CG, Santivasi WL, et al. Basic mechanisms of therapeutic resistance to radiation and chemotherapy in lung cancer. Cancer J 2013;19:200-7. [Crossref] [PubMed]
- West CM, Barnett GC. Genetics and genomics of radiotherapy toxicity: towards prediction. Genome Med 2011;3:52. [Crossref] [PubMed]
- Hydbring P, Badalian-Very G. Clinical applications of microRNAs. Version 3. F1000Res 2013;2:136. [PubMed]
- Metheetrairut C, Slack FJ. MicroRNAs in the ionizing radiation response and in radiotherapy. Curr Opin Genet Dev 2013;23:12-9. [Crossref] [PubMed]
- Metheetrairut C, Adams BD, Nallur S, et al. cel-mir-237 and its homologue, hsa-miR-125b, modulate the cellular response to ionizing radiation. Oncogene 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science 2001;294:862-4. [Crossref] [PubMed]
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993;75:843-54. [Crossref] [PubMed]
- Baek D, Villén J, Shin C, et al. The impact of microRNAs on protein output. Nature 2008;455:64-71. [Crossref] [PubMed]
- Selbach M, Schwanhäusser B, Thierfelder N, et al. Widespread changes in protein synthesis induced by microRNAs. Nature 2008;455:58-63. [Crossref] [PubMed]
- Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 2010;11:597-610. [PubMed]
- Ambros V. The functions of animal microRNAs. Nature 2004;431:350-5. [Crossref] [PubMed]
- Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell 2005;122:6-7. [Crossref] [PubMed]
- Turchinovich A, Weiz L, Burwinkel B. Extracellular miRNAs: the mystery of their origin and function. Trends Biochem Sci 2012;37:460-5. [Crossref] [PubMed]
- Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev 2013;27:31-9. [Crossref] [PubMed]
- Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh Migr 2007;1:156-8. [Crossref] [PubMed]
- Melo SA, Sugimoto H, O'Connell JT, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014;26:707-21. [Crossref] [PubMed]
- Kraemer A, Anastasov N, Angermeier M, et al. MicroRNA-mediated processes are essential for the cellular radiation response. Radiat Res 2011;176:575-86. [Crossref] [PubMed]
- Surova O, Akbar NS, Zhivotovsky B. Knock-down of core proteins regulating microRNA biogenesis has no effect on sensitivity of lung cancer cells to ionizing radiation. PLoS One 2012;7:e33134 [Crossref] [PubMed]
- Czochor JR, Glazer PM. microRNAs in cancer cell response to ionizing radiation. Antioxid Redox Signal 2014;21:293-312. [Crossref] [PubMed]
- Anastasov N, Höfig I, Vasconcellos IG, et al. Radiation resistance due to high expression of miR-21 and G2/M checkpoint arrest in breast cancer cells. Radiat Oncol 2012;7:206. [Crossref] [PubMed]
- Kraemer A, Barjaktarovic Z, Sarioglu H, et al. Cell survival following radiation exposure requires miR-525-3p mediated suppression of ARRB1 and TXN1. PLoS One 2013;8:e77484 [Crossref] [PubMed]
- He X, He L, Hannon GJ. The guardian's little helper: microRNAs in the p53 tumor suppressor network. Cancer Res 2007;67:11099-101. [Crossref] [PubMed]
- Zhang X, Wan G, Berger FG, et al. The ATM kinase induces microRNA biogenesis in the DNA damage response. Mol Cell 2011;41:371-83. [Crossref] [PubMed]
- Zhao L, Bode AM, Cao Y, et al. Regulatory mechanisms and clinical perspectives of miRNA in tumor radiosensitivity. Carcinogenesis 2012;33:2220-7. [Crossref] [PubMed]
- Hu H, Du L, Nagabayashi G, et al. ATM is down-regulated by N-Myc-regulated microRNA-421. Proc Natl Acad Sci U S A 2010;107:1506-11. [Crossref] [PubMed]
- Lal A, Pan Y, Navarro F, et al. miR-24-mediated downregulation of H2AX suppresses DNA repair in terminally differentiated blood cells. Nat Struct Mol Biol 2009;16:492-8. [Crossref] [PubMed]
- Crosby ME, Kulshreshtha R, Ivan M, et al. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res 2009;69:1221-9. [Crossref] [PubMed]
- Yan D, Ng WL, Zhang X, et al. Targeting DNA-PKcs and ATM with miR-101 sensitizes tumors to radiation. PLoS One 2010;5:e11397 [Crossref] [PubMed]
- Chun-Zhi Z, Lei H, An-Ling Z, et al. MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN. BMC Cancer 2010;10:367. [Crossref] [PubMed]
- Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle 2010;9:1072-83. [Crossref] [PubMed]
- Morgan WF, Sowa MB. Non-targeted effects induced by ionizing radiation: mechanisms and potential impact on radiation induced health effects. Cancer Lett 2015;356:17-21. [Crossref] [PubMed]
- Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res 2006;66:4795-801. [Crossref] [PubMed]
- Arscott WT, Tandle AT, Zhao S, et al. Ionizing radiation and glioblastoma exosomes: implications in tumor biology and cell migration. Transl Oncol 2013;6:638-48. [Crossref] [PubMed]
- Jella KK, Rani S, O'Driscoll L, et al. Exosomes are involved in mediating radiation induced bystander signaling in human keratinocyte cells. Radiat Res 2014;181:138-45. [Crossref] [PubMed]
- Al-Mayah A, Bright S, Chapman K, et al. The non-targeted effects of radiation are perpetuated by exosomes. Mutat Res 2015;772:38-45. [Crossref] [PubMed]
- Lehmann BD, Paine MS, Brooks AM, et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res 2008;68:7864-71. [Crossref] [PubMed]
- Mutschelknaus L, Peters C, Winkler K, et al. Exosomes Derived from Squamous Head and Neck Cancer Promote Cell Survival after Ionizing Radiation. PLoS One 2016;11:e0152213 [Crossref] [PubMed]
- Hazawa M, Tomiyama K, Saotome-Nakamura A, et al. Radiation increases the cellular uptake of exosomes through CD29/CD81 complex formation. Biochem Biophys Res Commun 2014;446:1165-71. [Crossref] [PubMed]
- de Jong OG, Verhaar MC, Chen Y, et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles 2012;1: [Crossref] [PubMed]
- Tang Y, Cui Y, Li Z, et al. Radiation-induced miR-208a increases the proliferation and radioresistance by targeting p21 in human lung cancer cells. J Exp Clin Cancer Res 2016;35:7. [Crossref] [PubMed]
- Beninson LA, Brown PN, Loughridge AB, et al. Acute stressor exposure modifies plasma exosome-associated heat shock protein 72 (Hsp72) and microRNA (miR-142-5p and miR-203). PLoS One 2014;9:e108748 [Crossref] [PubMed]
- Xu S, Wang J, Ding N, et al. Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect. RNA Biol 2015;12:1355-63. [Crossref] [PubMed]
- Yuan D, Xu J, Wang J, et al. Extracellular miR-1246 promotes lung cancer cell proliferation and enhances radioresistance by directly targeting DR5. Oncotarget 2016;7:32707-22. [PubMed]
- Hall JS, Taylor J, Valentine HR, et al. Enhanced stability of microRNA expression facilitates classification of FFPE tumour samples exhibiting near total mRNA degradation. Br J Cancer 2012;107:684-94. [Crossref] [PubMed]
- Drebber U, Lay M, Wedemeyer I, et al. Altered levels of the onco-microRNA 21 and the tumor-supressor microRNAs 143 and 145 in advanced rectal cancer indicate successful neoadjuvant chemoradiotherapy. Int J Oncol 2011;39:409-15. [PubMed]
- Wang XC, Du LQ, Tian LL, et al. Expression and function of miRNA in postoperative radiotherapy sensitive and resistant patients of non-small cell lung cancer. Lung Cancer 2011;72:92-9. [Crossref] [PubMed]
- Talwar S, House R, Sundaramurthy S, et al. Inhibition of caspases protects mice from radiation-induced oral mucositis and abolishes the cleavage of RNA-binding protein HuR. J Biol Chem 2014;289:3487-500. [Crossref] [PubMed]
- Hamama S, Noman MZ, Gervaz P, et al. MiR-210: A potential therapeutic target against radiation-induced enteropathy. Radiother Oncol 2014;111:219-21. [Crossref] [PubMed]
- Halimi M, Shahabi A, Moslemi D, et al. Human serum miR-34a as an indicator of exposure to ionizing radiation. Radiat Environ Biophys 2016;55:423-9. [Crossref] [PubMed]
- Yu Q, Li B, Li P, et al. Plasma microRNAs to predict the response of radiotherapy in esophageal squamous cell carcinoma patients. Am J Transl Res 2015;7:2060-71. [PubMed]
- Summerer I, Niyazi M, Unger K, et al. Changes in circulating microRNAs after radiochemotherapy in head and neck cancer patients. Radiat Oncol 2013;8:296. [Crossref] [PubMed]
- Summerer I, Unger K, Braselmann H, et al. Circulating microRNAs as prognostic therapy biomarkers in head and neck cancer patients. Br J Cancer 2015;113:76-82. [Crossref] [PubMed]
- Lanford RE, Hildebrandt-Eriksen ES, Petri A, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 2010;327:198-201. [Crossref] [PubMed]
- Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med 2013;368:1685-94. [Crossref] [PubMed]
- Cortez MA, Valdecanas D, Zhang X, et al. Therapeutic delivery of miR-200c enhances radiosensitivity in lung cancer. Mol Ther 2014;22:1494-503. [Crossref] [PubMed]
- Höfig I, Barth S, Salomon M, et al. Systematic improvement of lentivirus transduction protocols by antibody fragments fused to VSV-G as envelope glycoprotein. Biomaterials 2014;35:4204-12. [Crossref] [PubMed]


