
Pancreatic cancer cell invasion: mesenchymal switch or just hitchhiking?
For the majority of pancreatic cancer patients, metastatic spread is the most life-threatening issue, significantly shortening survival (1). Yet, the road for a cancer cell to successfully set its metastatic niche and to grow there is fortunately long and sown with pitfalls. Consistently, the estimated time for pancreatic cancer patients to develop a widely disseminated disease through subclonal metastatic evolution from a parental clone inside the primary carcinoma is 6.8-year, as recently reported (2). To the important question of whether the poor prognosis of pancreatic cancer is due to its late diagnosis, or because it metastasizes early during clonal evolution, Yachida et al. indeed answered that there is a long latency to development of an infiltrating cancer, and thus a large window of opportunity for early diagnosis and cure. Yet, until early detection of pancreatic cancer becomes routine, the reality is that most patients will likely continue to be diagnosed with advanced disease, as recently mathematically predicted through a comprehensive study that benefited from a large group of patient’s autopsy data (3).
Subclonal metastatic evolution implies gain of (epi)genetic heterogeneity that may arise from preexistent small populations of cancer stem cells that continuously give rise to genetically diverse populations of non-tumorigenic cells (4), as lately evidenced in pancreatic cancer (5). As epithelial-to-mesenchymal transition (EMT) seems a robust feature that arises during pancreatic cancer progression (6,7), even if recently reconsidered (8), the probability of generating, through the EMT program, highly tumorigenic cells capable of self-renewal and of repopulation of all phenotypic progeny present within the neoplasm (9), is plausible. The EMT is a reprogramming phase giving to epithelial cells not only features of stemness, but above all of motility and invasiveness, properties required to successfully accomplish their metastasis route. Mesenchymal traits are acquired at the expense of epithelial markers which are lost through transcriptional and/or epigenetic repression. Whether this phenotypic switch is irreversible, or subjected to constant reversibility considering that both epithelial and mesenchymal markers have been found in a same cell, is under intense debate (10). Oversimplification would be dangerous and one imagines that the picture is not unique, since (I) the high cellular and molecular heterogeneity of pancreatic tumor confers robust plasticity; (II) the stresses that pancreatic tumors are facing during their metastatic cascade or disease treatment history (e.g., stroma-derived, mechanical, hypoxia, genotoxic) select the most adapted subclones, and EMT seems a prerequisite. Nevertheless, the epithelial phenotype of metastases has shed the light on the reverse transition, i.e., mesenchymal-to-epithelial transition (MET), putatively occurring in the metastatic cancer cells that may have previously lost their epithelial phenotype to acquire metastatic skills, MET giving them back their proliferative properties required for efficient metastatic outgrowth.
EMT is driven by a family of transcription factors, mainly of ZEB, SNAIL and TWIST families, that in a multi-stage process remodel epithelial cell architecture, for the acquisition of increased cell migration, invasion and survival. Lineage tracing experiments in genetically engineered mouse models (GEMMs) have revealed that EMT occurs early during the pancreatic disease evolution (7). Indeed, circulating tumor cells with acquired mesenchymal traits enabling their migration/invasion into the blood flow, yet incapable of establishing metastases, are delaminated from the primary neoplasm as soon as precancerous lesions are present. Nevertheless, two recent studies on breast and pancreatic cancer suggested that EMT, although critical for tumor cells to acquire chemoresistance features, is dispensable for a tumor to metastasize (8,11). Those studies reopened the debate on the need “to be mesenchymal or not” during the cell metastatic cascade. Although Zheng et al. upon conditional deletion of the transcription factors twist or snail genes in a pancreatic cancer GEMM background, still observed as many metastases in those conditions as in the parental model, with metastatic cells keeping their epithelial features (E-cadherin expression) (8), one can envision that, in this model, partial EMT is present owing to compensatory mechanisms driving mesenchymal switch. Consistently, other transcription factors have been reported to do the job.
Recently, Heeg et al. reported that the expression of the ETS-transcription factor ETV1, which is essential for mesenchymal differentiation and stromal tissue identity during pancreatic branching morphogenesis, is progressively induced during the pancreatic ductal carcinogenesis sequence from preinvasive [pancreatic intraepithelial neoplasia (PanIN)] to invasive/metastatic lesions (12). Then, they showed that ETV1 is critical for stromal expansion and metastatic progression of pancreatic tumors. To do so, they used pancreatic ductal cells isolated from invasive lesions in GEMM (Pdx1Cre;KrasG12D/+; p53fl/+, named KPC), that could be traced owing to the expression of reporter proteins (YFP or dTomato), and in which they overexpressed ETV1. Orthotopic graft of ETV1-overexpressing cells in immunodeficient mice increased, as compared to parental mock cells, primary tumor growth via stroma expansion, and metastasis development. Interestingly, ETV1 overexpression provided to epithelial cells mesenchymal and metastatic features, in association with loss of E-cadherin expression, with upregulation of the matricellular protein SPARC expression, and with deposition of hyaluronic acid. The regulation by ETV1 was transcriptional, through direct activation of sparc and hyaluronic synthase 2 (has2) promoter activities. Interestingly, SPARC was expressed in YFP-positive tumor cells, suggesting a direct molecular link with ETV1. Sparc knockout in ETV1-overexpressing KPC cells functionally abrogated their potential, upon orthotopic graft in mice, to form stroma-rich tumors and to metastasize (although some SPARC was still detectable in the host stroma), placing SPARC as an autonomous functional target of ETV1 pro-metastatic effect in cancer cells. Altogether, these results place the transcription factor ETV1 as a novel driver of mesenchymal traits in pancreatic tumor cells, and tumor cell-derived SPARC as its direct functional effector. Whether this transcription factor is indeed a master regulator of stroma expansion and metastasis driven by KPC cells that endogenously present a 184-fold increase of ETV1, as compared to normal pancreatic ductal cells, is currently under investigation using conditional invalidation of etv1 in the KPC background. First results confirm that ETV1 is indeed involved but not as dramatically as expected, suggesting that activation of other pathway(s) may compensate for ETV1 absence. Unraveling one mechanism for hyaluronic acid production in pancreatic tumors (via HAS2 regulation by ETV1) is of high therapeutic interest since this matrix glycosaminoglycan is responsible for mechanic stresses that result in vascular collapse and subsequent reduced perfusion of these tumors (13). Enzymatic strategies aimed at alleviating hyaluronic acid to restore perfusion and help the diffusion of chemotherapies to tumor cells, are under clinical trials.
Redundancy of transcription factors that regulate the epithelial versus mesenchymal fate is multiple, suggesting that selection pressure during the subclonal metastatic evolution is high to keep this plasticity feature in the selected cancer clones. Among the panoply of transcription factors regulating EMT, the two major isoforms of the paired-related homeodomain transcription factor 1 (PRRX1), PRRX1a and PRRX1b, have recently been uncovered to play a role (14). Those transcription factors are up-regulated during ductal development, induction of acinar-to-ductal metaplasia, and evolution of PanINs. Strikingly, those splicing variants have opposite effects, i.e., EMT for PRRX1b (induction of invasion and tumor dedifferentiation), and MET for PRRX1a (stimulation of metastatic outgrowth in the liver and tumor differentiation), giving a first mechanistic information (through alternative splicing regulation between both isoforms) for the dynamic switch occurring between EMT and MET, observed in primary tumors vs. in metastases, respectively. The hepatocyte growth factor (HGF) was identified as a novel transcriptional target of PRRX1b explaining at least in part its mesenchymal-inducing effect, and therapeutic targeting of HGF in combination with gemcitabine was given as a promising strategy in a preclinical model of pancreatic cancer. Lastly, the transcription factor RUNX3 has been identified when comparing the expression profiles of non-metastatic versus metastatic pancreatic tumor cells that presented, in a KPC background, a mono-allelic loss of smad4/dpc4, versus wild-type or smad4/dpc4 bi-allelic loss, respectively (15). High RUNX3 expression explained cell differences in metastatic skills, mainly because RUNX3 can control both extracellular matrix (ECM) protein production (e.g., osteopontin, SPARC) and/or proliferation, depending on smad4/dpc4 status, facilitating cell seeding and/or growth in the primary tumor and metastatic niches. This study is consistent with the two phenotypes of pancreatic cancer patients that differ not in their morphologic differences at diagnosis but in their metastatic efficiencies for which inactivation of SMAD4, and as a result loss of SMAD4 protein expression, is a marker (16). Surprisingly in this study, metastatic potential in vivo, and migration or invasive skills in vitro, were not reminiscent of E-cadherin expression loss, yet a conventional marker of EMT. These results suggested that loss of E-cadherin expression is not a sufficient marker to monitor “complete functional EMT”, and that cell invasion and metastasis can co-exist with persistence of the epithelial phenotype (e.g., high E-cadherin expression).
Consistently, the induction of EMT with downregulation of E-cadherin expression is likely tunable, dependent on whether complete or partial EMT signaling is present. The EMT program is thought to be controlled by the tumor microenvironment, including by soluble factors released by stromal cells [e.g., cancer-associated fibroblasts (CAFs) (17,18) or tumor-associated macrophages (19,20)], mechanical (tumor stiffening) or metabolic stresses (hypoxia) (21), which locally downregulate epithelial characteristics and facilitate cell escape from the primary tumor. However, with local upstream signaling lost, cells undergo MET reversion after metastatic seeding in the secondary organ (10). Yet likely indispensable during the metastatic process, the observed differences in EMT reprogrammation, [i.e., induced in a cell-autonomous fashion through irreversible mesenchymal phenotype acquisition, or stroma-induced tunable and localized phenotypic switch (e.g., in leading cells of the invasive fronts)], may explain also the large differences observed in cell migration, individual in undifferentiated tumors, or collective in differentiated tumors where CAFs certainly help epithelial cancer cell clusters to crawl into the stroma (22) (Figure 1). These types of migration eventually co-exist in different regions of a same tumor, and are plastic such as during therapeutic stresses, probably explaining the difficulty to therapeutically target the metastatic process (23).
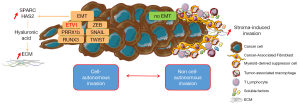
Nevertheless a common feature of cancer invading cells is their entwined required relationship with the stroma, that they may either directly produce through EMT reprogrammation, as described by Heeg et al. (12), or interact with during collective yet highly efficient migration in which EMT may not be necessary.
Acknowledgments
The authors would like to thank Servier Medical Art for their image bank used to create the illustrations.
Funding: CRCT team 6 is supported by LNCC (Ligue Nationale Contre le Cancer RAB09006BBA), the Canceropole Grand Sud-Ouest (RMA04002BPA), the ANR (Agence Nationale pour la Recherche, project n°R06423BS), the Labex TOUCAN, the Fondation de France (2014 00047506), and the French National Institute of Cancer (INCa, PLBIO13-134 and PLBIO15-115). R Samain salary is funded by Fondation pour la Recherche Médicale (FRM40493).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xiaotian Sun (Department of Internal Medicine, Clinic of August First Film Studio, Beijing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yachida S, Iacobuzio-Donahue CA. Evolution and dynamics of pancreatic cancer progression. Oncogene 2013;32:5253-60. [Crossref] [PubMed]
- Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114-7. [Crossref] [PubMed]
- Haeno H, Gonen M, Davis MB, et al. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell 2012;148:362-75. [Crossref] [PubMed]
- Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell 2012;21:283-96. [Crossref] [PubMed]
- Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res 2007;67:1030-7. [Crossref] [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [Crossref] [PubMed]
- Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012;148:349-61. [Crossref] [PubMed]
- Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015;527:525-30. [Crossref] [PubMed]
- Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704-15. [Crossref] [PubMed]
- Diepenbruck M, Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol 2016;43:7-13. [Crossref] [PubMed]
- Fischer KR, Durrans A, Lee S, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015;527:472-6. [Crossref] [PubMed]
- Heeg S, Das KK, Reichert M, et al. ETS-Transcription Factor ETV1 Regulates Stromal Expansion and Metastasis in Pancreatic Cancer. Gastroenterology 2016;151:540-553.e14. [Crossref] [PubMed]
- Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:418-29. [Crossref] [PubMed]
- Reichert M, Takano S, von Burstin J, et al. The Prrx1 homeodomain transcription factor plays a central role in pancreatic regeneration and carcinogenesis. Genes Dev 2013;27:288-300. [Crossref] [PubMed]
- Whittle MC, Izeradjene K, Rani PG, et al. RUNX3 Controls a Metastatic Switch in Pancreatic Ductal Adenocarcinoma. Cell 2015;161:1345-60. [Crossref] [PubMed]
- Blackford A, Serrano OK, Wolfgang CL, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res 2009;15:4674-9. [Crossref] [PubMed]
- Moatassim-Billah S, Duluc C, Samain R, et al. Anti-metastatic potential of somatostatin analog SOM230: Indirect pharmacological targeting of pancreatic cancer-associated fibroblasts. Oncotarget 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Duluc C, Moatassim-Billah S, Chalabi-Dchar M, et al. Pharmacological targeting of the protein synthesis mTOR/4E-BP1 pathway in cancer-associated fibroblasts abrogates pancreatic tumour chemoresistance. EMBO Mol Med 2015;7:735-53. [Crossref] [PubMed]
- Harney AS, Arwert EN, Entenberg D, et al. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer Discov 2015;5:932-43. [Crossref] [PubMed]
- Karnevi E, Rosendahl AH, Hilmersson KS, et al. Impact by pancreatic stellate cells on epithelial-mesenchymal transition and pancreatic cancer cell invasion: Adding a third dimension in vitro. Exp Cell Res 2016;346:206-15. [Crossref] [PubMed]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15:178-96. [Crossref] [PubMed]
- Gaggioli C. Collective invasion of carcinoma cells: when the fibroblasts take the lead. Cell Adh Migr 2008;2:45-7. [Crossref] [PubMed]
- Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 2011;147:992-1009. [Crossref] [PubMed]


