
A vicious cycle of metabolic interaction between leukemia stem cells and adipose tissues: implications in cachexia and drug resistance
Compelling evidences from recent studies suggest that leukemia stem cells (LSCs), a small subpopulation of malignant cells with long-term self-renewal capacity, play a key role in leukemia development, drug resistance, and disease relapse and recurrence (1). It has also been known for some time that tumor microenvironment or tissue niches may exert profound effect on the fates and viability of LSCs. A recent study by Ye et al. revealed a metabolic interaction between LSCs and adipose tissues, and showed that LSCs could be segregated into two metabolically and functionally distinct subgroups by their expression of the fatty acid (FA) transporter CD36 (2). The authors also suggested that gonadal adipose tissue (GAT) might provide a preferred niche to support CD36+ LSCs and promote drug resistance in a mouse model of blast crisis chronic myeloid leukemia (CML).
Leukemia is a group of clonal and heterogeneous hematological malignancies. In 1994, Lapidot et al. reported cells capable of initiating acute myeloid leukemia (AML) after transplantation into immune-deficient mice and proposed the concept of LSCs (3). Since then, many studies have demonstrated the presence of LSCs that are able to initiate and maintain leukemic disease, and started to identify biomarkers for LSCs and to explore the possibility to target LSCs as a therapeutic approach for the cure of leukemia (4,5). Base on the surface expression of CD34+ and CD38−, additional molecules including CD123, CD32, CD33, CD47, CD96, CD99, IL1RAP and TIM-3 have been proposed as LSCs markers (6). More recent data demonstrated that both intrinsic and extrinsic components are crucial to influence the survival and fates of LSCs. Intrinsic factors include regulators of cell cycle and pro-survival pathways, factors that regulate oxidative stress, and special molecular components that promote self-renewal. Extrinsic components include certain chemokine receptors, adhesion molecules, hypoxia-related factors, nutrients and certain metabolic intermediates in the tissue microenvironment. LSCs reside in specific niches and their stemness and functional status are dependent on a complex interplay of the extrinsic and intrinsic factors, which together govern the overall fate of LSCs (7).
Epidemiological data suggest a significant association between increased body mass index (BMI) and hematological neoplasms. Several large cohort studies, including meta-analyses of cohorts and case control studies, have revealed an association between high incidence of leukemia and overweight, suggesting that obesity is a poor prognostic factor for leukemia (8). The metabolic, endocrinologic, immunologic and inflammatory-like abnormalities resulting from obesity may increase the risk of cancer (9). However, how obesity affects leukemia cells still remains unclear. Han et al. found that the stromal vascular fraction (SVF) in adipose tissue (AT) contains phenotypic and functional hematopoietic stem cells (HSCs), and showed that AT might be a possible extramedullary reservoir and niche for HSCs (10). These observations led to a postulation that AT might provide a favorable environment for the survival and growth of LSCs. Using a murine model of blast crisis chronic myeloid leukemia (bcCML) as an experimental model, Ye and colleagues found that GAT indeed served as a reservoir for LSCs (2). In leukemic GAT tissue microenvironment, LSCs may secret pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1 alpha (IL-1α), interleukin-1 beta (IL-1β) and CSF2. These pro-inflammatory cytokines may then in turn stimulate lipolysis in the adipose tissue, leading to an elevation of serum free fatty acids (FFAs), atrophy of GAT, and loss of body weight. Moreover, FFAs from lipolysis may provide metabolic fuel for β-oxidation in LSCs and further exacerbate inflammation-induced lipolysis. These findings suggest that LSCs may utilize GAT as a niche to support their metabolism by disrupting lipid metabolic balance in GAT through paracrine signaling of pro-inflammatory cytokines to enhance lipolysis, which further support the metabolism of LSCs. This metabolic interaction between LSCs and adipose tissues seems to form a vicious cycle of lipolysis and inflammation (Figure 1). This might be a potential mechanism contributing to abnormal lipid consumption and cachexia.
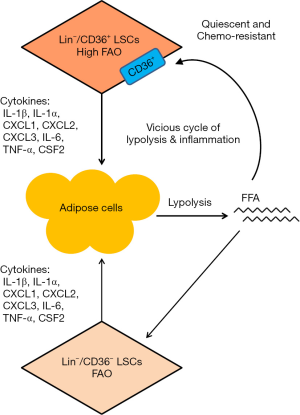
Ye et al. also showed that LSCs in adipose tissue were heterogeneous, and could be classified into two distinct subgroups by their expression of CD36 (2). Cluster of differentiation 36 (CD36) is an 88-kDa transmembrane glycoprotein present in a wide variety of cell types. Its main function is to facilitate the update of FA into the cells. Specifically, CD36 enable the transport of non-esterified fatty acids (NEFA), which are the products of lipolysis of triglyceride-rich lipoproteins such as very low density lipoprotein (VLDL), across plasma membranes into cells (such as monocytes, cardiomyocytes, and adipocytes) for use in fatty acid β-oxidation (FAO) and lipid deposition (11). The biological consequences of CD36 expression in tumor cells are somewhat controversial. Some studies suggest that a high expression of CD36 could promote endothelial cell apoptosis and reduce tumor metastasis in vivo, and a loss of CD36 expression seems to correlate with tumor progression and increased metastatic potential (12,13). However, other studies suggest that expression of CD36 seems to be an indicator of tumor cell dissemination in chronic lymphocytic leukemia (CLL) and in acute myelocytic leukemia (14,15). Little was known about CD36 expression in LSCs and its functional significance. Ye et al. showed that CD36 was not expressed in normal HSCs, but was highly expressed in a subset of LSCs. The expression of CD36 in LSCs could promote the cellular uptake of fatty acids and enhance their utilization through β-oxidation. Interestingly, the cellular ATP content was lower in CD36+ LSCs despite higher β-oxidation for a yet unknown reason. Another surprising finding was that CD36+ LSCs were more sensitive to inhibition of glycolysis by 2-deoxyglucose (2-DG) and more resistant to the inhibition of mitochondrial respiratory complex I by rotenone. If CD36+ LSCs were more effective in using FA as an energy source through active β-oxidation, these cells would be expected to be less dependent on glycolysis to generate ATP and more sensitive to inhibition of mitochondrial respiration, a necessary process to generate ATP downstream of β-oxidation in the mitochondria. Obviously, the complex energy metabolism in LSCs and their dependency on glucose and/or fatty acids still require further investigation.
Leukemia stem cells are in general less sensitive to chemotherapeutic agents compared to the bulk of non-stem leukemia cells. This was confirmed in Ye et al. (2). One important finding from their study was that CD36+ LSCs in adipose tissues were more resistant to multiple chemotherapeutic drugs compared to CD36− LSCs. The authors also showed that a loss of CD36 expression decreased leukemic burden in GAT and sensitized LSCs to chemotherapy (2). The mechanisms by which CD36 confers drug resistance still remain unclear. One possible explanation was that CD36+ cells were more quiescent in nature and thus were less sensitive to cell-cycle specific chemotherapeutic agents. However, it is unclear how such phenomenon could mechanistically link to the active uptake of FA and β-oxidation conferred by CD36 expression in LSCs.
The work by Ye et al. has revealed a complex metabolic interaction between LSCs and adipose tissues where LSCs promote lipolysis through secretion of inflammatory cytokines, and the FFAs generated from lipolysis in turn fuel the metabolism and survival of LSCs. The expression of CD36 in a subset of LSCs further enhances the uptake of fatty acids and lipid metabolism, and is associated with drug resistance in this subset of LSCs. These complex interactions seem to form a vicious cycle, potentially leading to cancer cachexia and drug resistance (Figure 1). The results of this study have potentially important clinical implications in developing more effective therapeutic strategies to eliminate drug-resistant LSCs and overcome drug resistance by targeting CD36 and its relevant pathways. It may also be possible to develop new intervention strategies to prevent cachexia associated metabolic abnormalities induced by LSCs in adipose tissues. However, since the underlying mechanisms responsible for drug resistance in CD36+ LSCs still remain unclear and the reason for LSCs with highly active β-oxidation being more sensitive to glycolytic inhibition and more resistant to inhibition of mitochondrial respiration still remain poorly understood, further study in this important area is required in order to develop mechanism-based therapeutic strategies. In addition, because acute lymphocytic leukemia (ALL) has a poor prognosis in adult and tends to infiltrate GAT such as testis (16), it would be important to extend this study to ALL. A recent study suggests that T-ALL cells have dynamic interactions with bone marrow stromal microenvironments, leading to an accumulation of disease burden and a rapid remodeling of the bone marrow microenvironment (17). It would be important to test if altered lipid metabolism is involved during this disease progression.
Acknowledgments
Funding: This work was supported in part by grants from State Scholarship Fund of China and National Natural Sciences Foundation of China (No. 81502573) to P-P.L.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Chen Qian (Center for Inflammation & Epigenetics, Houston Methodist Hospital Research Institute, Houston, TX, USA).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.43). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hackl H, Steinleitner K, Lind K, et al. A gene expression profile associated with relapse of cytogenetically normal acute myeloid leukemia is enriched for leukemia stem cell genes. Leuk Lymphoma 2015;56:1126-8. [Crossref] [PubMed]
- Ye H, Adane B, Khan N, et al. Leukemic Stem Cells Evade Chemotherapy by Metabolic Adaptation to an Adipose Tissue Niche. Cell Stem Cell 2016;19:23-37. [Crossref] [PubMed]
- Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994;367:645-8. [Crossref] [PubMed]
- Lee GY, Kim JA, Oh IH. Stem cell niche as a prognostic factor in leukemia. BMB Rep 2015;48:427-8. [Crossref] [PubMed]
- Won EJ, Kim HR, Park RY, et al. Direct confirmation of quiescence of CD34+CD38- leukemia stem cell populations using single cell culture, their molecular signature and clinicopathological implications. BMC Cancer 2015;15:217. [Crossref] [PubMed]
- Ravandi F, Estrov Z. Eradication of leukemia stem cells as a new goal of therapy in leukemia. Clin Cancer Res 2006;12:340-4. [Crossref] [PubMed]
- Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol 2011;29:591-9. [Crossref] [PubMed]
- Lichtman MA. Obesity and the risk for a hematological malignancy: leukemia, lymphoma, or myeloma. Oncologist 2010;15:1083-101. [Crossref] [PubMed]
- Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer 2011;11:886-95. [Crossref] [PubMed]
- Han J, Koh YJ, Moon HR, et al. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood 2010;115:957-64. [Crossref] [PubMed]
- Bharadwaj KG, Hiyama Y, Hu Y, et al. Chylomicron- and VLDL-derived lipids enter the heart through different pathways: in vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J Biol Chem 2010;285:37976-86. [Crossref] [PubMed]
- Koch M, Hussein F, Woeste A, et al. CD36-mediated activation of endothelial cell apoptosis by an N-terminal recombinant fragment of thrombospondin-2 inhibits breast cancer growth and metastasis in vivo. Breast Cancer Res Treat 2011;128:337-46. [Crossref] [PubMed]
- DeFilippis RA, Chang H, Dumont N, et al. CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer Discov 2012;2:826-39. [Crossref] [PubMed]
- Rutella S, Rumi C, Puggioni P, et al. Expression of thrombospondin receptor (CD36) in B-cell chronic lymphocytic leukemia as an indicator of tumor cell dissemination. Haematologica 1999;84:419-24. [PubMed]
- Perea G, Domingo A, Villamor N, et al. Adverse prognostic impact of CD36 and CD2 expression in adult de novo acute myeloid leukemia patients. Leuk Res 2005;29:1109-16. [Crossref] [PubMed]
- Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol 2011;29:532-43. [Crossref] [PubMed]
- Hawkins ED, Duarte D, Akinduro O, et al. T-cell acute leukaemia exhibits dynamic interactions with bone marrow microenvironments. Nature 2016;538:518-22. [Crossref] [PubMed]


