Megestrol acetate in cancer patients with anorexia-cachexia syndrome: a meta-analysis
Introduction
Anorexia-cachexia syndrome (ACS) is a common clinical problem that substantially impacts upon the quality of life and survival of affected patients. It is characterised by loss of appetite, weight loss and tissue wasting, accompanied by a decrease in muscle mass and adipose tissue, impoverishing quality of life and often preceding the patient’s death (1,2).
More than two-thirds of patients dying from advanced cancer suffer from anorexia-cachexia syndrome (3). ACS is also described in other pathologies such as in acquired immune deficiency syndrome (AIDS), anorexia nervosa, degenerative illnesses of the central nervous system and terminally ill patients (4). Incidence is variable and difficult to determine but in general the syndrome may occur in 15% to 40% of patients with cancer, and in more than 80% of patients with advanced diseases (5).
Megestrol acetate (MA) is a synthetic hormone (progestogen) used for the therapy of hormone-dependent cancer, mainly endometrial cancer and less commonly breast cancer. This drug is also used for symptom relief in cancer patients with ACS. We therefore performed a meta-analysis of the MA clinical trial experience to ascertain whether administration of MA relieved the ACS in patients with cancer.
Materials and methods
Publication search
The electronic databases PubMed and Web of Science were searched for studies to include in the present meta-analysis. An upper date limit of April 01, 2013 was applied; we used no lower date limit. Keywords included in our search were “neoplasm”, “cancer”, “cachexia”, “anorexia”, “megestrol acetate” and was limited to “randomized controlled clinical trials”. Abstracts and virtual meeting presentations containing the term “megestrol acetate” and “cancer” from the American Society of Clinical Oncology conferences (http://www.asco.org/ASCO) between January 2000 and Dec 2012 were also referenced to identify relevant clinical trials. Our initial selection of articles relied on careful reading of abstracts. We also reviewed the Cochrane Library for relevant articles. The references reported in the identified studies were also used to complete the search. When the same patient population was used in several publications, only the most recent, largest or complete study was included in this meta-analysis.
Study selection
The goal of this study was to evaluate the effect of MA for the treatment of cancer patients with ACS. The Primary outcomes for the magnitude of benefit analysis were weight gain and appetite improvement. Therefore, we selected for analysis only those randomized clinical trials that directly compared patients with cancer treated with and without MA. Phase I and single-arm phase II trials were excluded due to their lack of control groups. Specifically, clinical trials that met the following criteria were included in the meta-analysis: prospective phase II and III randomised clinical trials in patients with cancer; random assignment of participants to MA treatment or control/placebo in addition to concurrent chemotherapy and/or radiotherapy; and available data including weight gain and appetite improvement. Trials with uncertain or marked inequality of characteristics between groups at baseline were also excluded. Two reviewers (P.Z and Q.W) independently determined study eligibility. Disagreements were resolved by consensus.
Quality assessment
An open assessment of the trials was performed using the methods reported by Jadad and colleagues (6), which assessed the trials according to the following three questions: (I) whether reported an appropriate randomization method (0-2 scores); (II) whether reported an appropriate blinding method (0-2 scores); (III) whether reported withdrawals and dropouts (0-1 score).
Data extraction
All the data were independently abstracted by two investigators (P.Z., Q.Q.) according to the inclusion criteria listed above. Disagreements were resolved by discussing with an independent expert (L.Y.). The following information were sought from each paper, although some papers did not contain all of them: first author, year of publication, number of patients, treatment information, concurrent treatment and quality scores according to Jadad methods.
Statistical analysis
The overall the relative risks (RRs) for weight gain/appetite improvement and 95% confidence intervals (CIs) were calculated using Reviewer Manager Version 5.0 provided by the Cochrane Collaboration (7). For the meta-analysis, we used fixed-effects (weighted with inverse variance) or random effects model (8). For each meta-analysis, the Cochran’s Q statistic and I2 score were first calculated to assess the heterogeneity among the proportions of the included trials (9). For the P value of Cochran’s Q statistic <0.1, the assumption of homogeneity was deemed invalid, and a random-effects model was reported. The causes of heterogeneity were also explored in this context. Otherwise, results from the fixed-effects model were reported. A two-tailed P value <0.05 was judged as statistically significant. We used the Begg’s and Egger’s tests to determine the presence of publication bias (10,11). A two-tailed P value of <0.05 was considered statistically significant.
Results
Study characteristics
Our search yielded a total of 132 potentially relevant clinical studies on MA and treatment of cancer patients with ACS in the literature. After excluding review articles, phase I studies, single-arm phase II studies, case reports, meta-analyses and observational studies, 11 phase II-III randomized controlled clinical trials (12-22) were included in our meta-analysis. Table 1 presents the principal characteristics of these studies. The dose of MA treatment ranged form 160 to 800 mg/d. Concomitant treatment varied between trials as follows: chemotherapy (4 trials), chemoradiotherapy (2 trials), palliative radiotherapy (2 trial) and treatment not reported (5 trials).
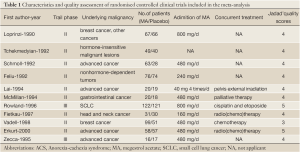
Full table
The effect of MA for weight gain
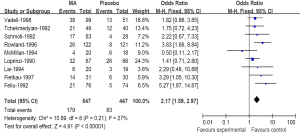
A meta-analysis was performed to calculate the overall RR of weight gain associated with MA in comparison with controls for 9 trials included 994 patients. These trials identified a significantly increased risk of weight gain among patients treated with MA (179 events among 534 patients treated with MA vs. 83 events among 447 control patients; RR 2.17; 95% CI: 1.59-2.97) (Figure 1), suggesting a 117% greater risk for weight gain with MA compared with a control. There was no significant heterogeneity when evaluating all 9 trials (heterogeneity: Chi2=10.08; I2=27%; P=0.21).
The effect of MA for appetite improvement
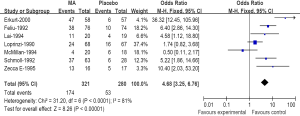
A meta-analysis was performed to calculate the overall RR of appetite improvement associated with MA in comparison with controls for 7 trials included 601 patients. These trials identified a significantly increased risk of appetite improvement among patients treated with MA (174 events among 321 patients treated with MA vs. 53 events among 280 control patients; RR 4.68; 95% CI: 3.25-6.76) (Figure 2), suggesting a 368% greater risk for appetite improvement with MA compared with a control. There was significant heterogeneity when evaluating all 7 trials (heterogeneity: Chi2=31.2; I2=81%; P=0.001).
Publication bias
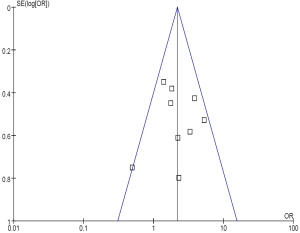
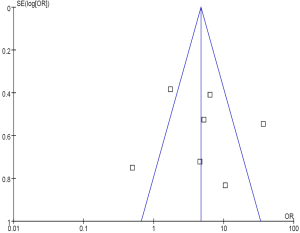
No evidence of publication bias was detected for the primary end point of this study by either the Begg or Egger test (Begg test, P=0.43; Egger test, P=0.59) (Figures 3,4).
Discussion
An international consensus statement defines cachexia as weight loss greater than 5%, or weight loss greater than 2% in individuals already showing depletion according to current body weight and height [body mass index (BMI) <20 kg/m2]or skeletal muscle mass (sarcopaenia) (23). The mechanism that causes cachexia is poorly understood, but inflammatory cytokine s probably have a role, such as tumour necrosis factor-alpha (which is al so nickname d “cachexin” or “cachectin”), angiotensin II and glucocorticoids, interferon gamma and interleukin 6, as well as the tumour-secreted proteolysis-inducing factor (24). Ghrelin levels are also high in patients who have cancer-induced cachexia (25).
In our meta-analysis, we involved 11 RCTs including a total of 1,142 patients with cancer and included for meta-analysis. Cancer patients with ACS who received MA had increased weight gain (179 events among 534 patients treated with MA vs. 83 events among 447 control patients; RR 2.17; 95% CI: 1.59-2.97), and increased appetite improvement (174 events among 321 patients treated with MA vs. 53 events among 280 control patients; RR 4.68; 95% CI: 3.25-6.76). The presented systematic review and the attempt at summarizing quantitatively the results did not bring unexpected conclusions. Similarly to the previously published meta-analysgs (26-28), an appetite improvement shown in absolute values (and weight gain can be noticed. In the previously published meta-analyses comparable results regarding weight gain [RR 2.16; 95% CI: 1.45-3.21 and relative benefit (RB) 2.14; 95% CI: 1.41-3.24] (27,28), appetite improvement (RR 2.33; 95% CI: 1.52-3.59 and RB 3.03; 95% CI: 1.83-5.01) were obtained.
Early intervention and attention to nutritional status are essential in patients with anorexia-cachexia syndrome. Pharmacological interventions for neoplastic cachexia include drugs that stimulate the appetite: megestrol acetate (MA) and dronabinol; cy tokine inhibitors [such as cyproheptadine, thalidomide, pentoxifylline and an eicosape ntaenoic acid (EPA)]; and anabolic agents such as nandrolone decanoate, oxandrol one and corticosteroids (29). EPA seems to suppress well -characterised me diators of cancer-associated wasting, including interleukin-6, an inflammatory cytokine. It al so acts over the proteolysis-inducing factor, another well-described mediator (30,31).
MA is a synthetic progestogen agent. It was first synthesized in England in 1963. Developed as an oral contraceptive, the agent was first tested in the treatment of breast cancer in 1967 and, later on, f or the treatment of endometrial cancer. MA is currently used to improve appetite and to increase weight in cancer-associated anorexia. From 1993, MA was approved by the Food and Drug Administration (FDA) in the USA for the treatment of anorexia, cachexia or unexplained weight loss in patients with AIDS. In addition, there are recent reports of the drug being used to improve the quality of life of elderly patients with cachexia.
In conclusion, our study has shown that the MA is associated with a significantly increased weight gain and appetite improvement in cancer patients with ACS. Because of a low value of available studies, for a more reliable assessment of MA efficacy in cancer-associated ACS, it is necessary to perform a randomized controlled trial of high methodological quality.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.04.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nelson KA, Walsh D, Hussein M. A phase II study of low-dose megestrol acetate using twice-daily dosing for anorexia in nonhormonally dependent cancer. Am J Hosp Palliat Care 2002;19:206-10. [PubMed]
- Splinter TA. Cachexia and cancer: a clinician’s view. Ann Oncol 1992;3:25-7. [PubMed]
- Argilés JM, Meijsing SH, Pallarés-Trujillo J, et al. Cancer cachexia: a therapeutic approach. Med Res Rev 2001;21:83-101. [PubMed]
- Von Roenn JH, Knopf K. Anorexia/cachexia in patients with HIV: lessons for the oncologist. Oncology (Williston Park) 1996;10:1049-56; discussion 1062-4, 1067-8.
- Bruera E. Clinical management of anorexia and cachexia in patients with advanced cancer. Oncology 1992;49:35-42. [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [PubMed]
- Review Manager (RevMan) [Computer program]. Version 5.0. Copenhagen: the nordic cochrane centre, the cochrane collaboration, 2008.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Loprinzi CL, Ellison NM, Schaid DJ, et al. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer Inst 1990;82:1127-32. [PubMed]
- Tchekmedyian NS, Hickman M, Siau J, et al. Megestrol acetate in cancer anorexia and weight loss. Cancer 1992;69:1268-74. [PubMed]
- Feliu J, González-Barón M, Berrocal A, et al. Usefulness of megestrol acetate in cancer cachexia and anorexia. A placebo-controlled study. Am J Clin Oncol 1992;15:436-40. [PubMed]
- Schmoll E. Risks and benefits of various therapies for cancer anorexia. Oncology 1992;49:43-5. [PubMed]
- Lai YL, Fang FM, Yeh CY. Management of anorexic patients in radiotherapy: a prospective randomized comparison of megestrol and prednisolone. J Pain Symptom Manage 1994;9:265-8. [PubMed]
- McMillan DC, Simpson JM, Preston T, et al. Effect of megestrol acetate on weight loss, body composition and blood screen of gastrointestinal cancer patients. Clin Nutr 1994;13:85-9. [PubMed]
- Zecca E, Martini C, Venturino P, et al. Efficacy of megestrol acetate on anorexia in patients with advanced non hormone-related tumors: a double-blind placebo controlled clinical trial. Eur J Cancer 1995;31:S261-S262.
- Rowland KM Jr, Loprinzi CL, Shaw EG, et al. Randomized double-blind placebo-controlled trial of cisplatin and etoposide plus megestrol acetate/placebo in extensive-stage small-cell lung cancer: a North Central Cancer Treatment Group study. J Clin Oncol 1996;14:135-41. [PubMed]
- Fietkau R, Riepl M, Kettner H, et al. Supportive use of megestrol acetate in patients with head and neck cancer during radio(chemo)therapy. Eur J Cancer 1997;33:75-9. [PubMed]
- Vadell C, Seguí MA, Giménez-Arnau JM, et al. Anticachectic efficacy of megestrol acetate at different doses and versus placebo in patients with neoplastic cachexia. Am J Clin Oncol 1998;21:347-51. [PubMed]
- Erkurt E, Erkisi M, Tunali C. Supportive treatment in weight-losing cancer patients due to the additive adverse effects of radiation treatment and/or chemotherapy. J Exp Clin Cancer Res 2000;19:431-9. [PubMed]
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [PubMed]
- Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 2009;89:381-410. [PubMed]
- Wolf I, Sadetzki S, Kanety H, et al. Adiponectin, ghrelin, and leptin in cancer cachexia in breast and colon cancer patients. Cancer 2006;106:966-73. [PubMed]
- Pascual López A, Roqué i Figuls M, Umjtia Cuchi G, et al. Systemat-ic review of megestrol acetate in the treatment of anorexia-cachexia syn-drome. J Pain Symptom Manage 2004;27:360-9. [PubMed]
- Maltoni M, Nanni O, Scarpi E, et al. High-dose progestins for the treat-ment of cancer anorexia-cachexia syndrome: a systematic review of ran-domized clinical trials. Ann Oncol 2001;12:289-300. [PubMed]
- Berenstein EG, Ortiz Z. Megestrol acetate for treatment of anorexia-cacbexia syndrome. Cochrane Database Syst Rev 2005;CD004310 [PubMed]
- Balog DL, Epstein ME, Amodio-Groton MI. HIV wasting syndrome: treatment update. Ann Pharmacother 1998;32:446-58. [PubMed]
- Barber MD, Ross JA, Voss AC, et al. The effect of an oral nutritional supplement enriched with fish oil on weight-loss in patients with pancreatic cancer. Br J Cancer 1999;81:80-6. [PubMed]
- Wigmore SJ, Fearon KC, Maingay JP, et al. Down-regulation of the acute-phase response in patients with pancreatic cancer cachexia receiving oral eicosapentaenoic acid is mediated via suppression of interleukin-6. Clin Sci (Lond) 1997;92:215-21. [PubMed]