
Boron neutron capture therapy for brain tumors
Introduction
Glioblastoma (GBM) is a common malignant brain tumor in adults, and many recur within several months and show fatal progression within 2 years after the initial treatment. Extensive resection of the contrast-enhancing part of a tumor under image-guided surgery using fluorescence with 5-aminolevulinic acid, neuronavigation, and intraoperative magnetic resonance imaging (MRI) is shown to be beneficial for prolongation of the post-operative survival time (1,2). Aggressive cyto-reductive surgery is not indicated for the tumor in the eloquent brain. Invading cells are evident at distances of 2 to 3 cm or even further from the main tumor mass of GBM, which can be clinically identified by the contrast enhancement area on a magnetic resonance image. Thus, post-operative adjuvant therapies are essential for the treatment of post-surgical residual tumor mass and microscopic invading tumor cells in the patients with GBM.
Among several chemotherapeutic agents for malignant glioma (3,4), the effectiveness of temozolomide or carmustine wafers has been shown. For example, the EORTC clinical trial provided Class I evidence that the concomitant and adjuvant use of temozolomide with the conventional radiotherapy leads to a modest but significant survival advantage (median survival time, or MST: 14.6 mos) compared to the conventional radiotherapy alone (MST 12.1 mos), approximately with 25% of the patients surviving longer than 24 mos (5).
Two prospective studies provided Class II evidence and also showed modest benefits of carmustine wafers for GBM patients (4). In the report of Westphal et al. (6), a subanalysis of 207 GBM patients showed that the carmustine wafer group had a longer mean survival (13.5 mos) than the placebo group (11.4 mos). In a study by Valtonen et al. (7), regarding the survival of the 27 GBM patients among the whole series of 32 patients, the group that received carmustine wafers had a longer mean survival (53.3 wks) than the placebo wafer group (39.9 wks). Because of the limited benefits produced by standard (conventional) radiotherapy and chemotherapy to date, there has been also significant interest in new entity of radiotherapy and targeted molecular agents for the treatment of GBM.
Dose escalation studies using conventional X-ray fractionation, stereotactic radiosurgery, fractionated proton beam radiation, or other conformal radiotherapies have shown median survival times which vary from 9.5 to 26 mos (8,9). These studies and their failure analyses imply that at least 90 Gy must be delivered to achieve local control of GBM. Such a high-dose of radiation exceeds the accepted tolerance of normal brain tissue. Thus, high-dose radiation must be delivered with the upmost selectivity for tumor cells, to minimize radiation damage to the surrounding normal brain. Tumor-cell selectivity at the microscopic level is thus desirable. BNCT has been indicated primarily for GBM because of the theoretical selective sterilization of microscopic invading cells in the brain.
Boron neutron capture therapy (BNCT)
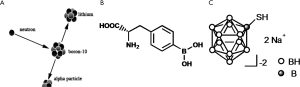
Boron neutron capture therapy (BNCT) has been proposed to provide tumor cell-selective high-linear energy transfer particle radiotherapy. The nuclear reaction between boron-10 (10B) and thermal neutrons releases high LET α and 7Li particles through the boron neutron capture reaction, 10B(n, α) 7Li (Figure 1). The very short path length (<9 µm) of α-particles and 7Li enables high-LET irradiation of 10B-loaded tumor cells, minimizing undesirable damage to 10B-unloaded normal cells. The effectiveness of BNCT is highly dependent on the amount of these particles and the selectivity of the boron compound in tumor cells. In BNCT clinical study, the minimum tumor dose of gross tumor volume (GTV) was around 30 Gy (10).
Although low-energy thermal neutrons (<0.53 eV) are captured most efficiently by 10B nuclei, the shallow penetration limits their usefulness. For external beam BNCT, it is essential to use epithermal neutrons, which lose energy during the penetration of normal tissue (e.g., skin, cranium) and convert to thermal neutrons. Most commonly in BNCT for brain tumors, epithermal neutron beam irradiation is performed at a research reactor, and in a single fraction (Figure 2).
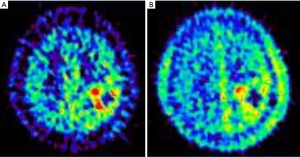
To deliver 10B, two boron drugs, p-dihydroxyboryl-phenylalanine (BPA) and sulfhydryl borane Na2B12H11SH (BSH), are currently available for BNCT clinical studies (Figure 1). Positron emission tomography (PET) is used to estimate the 10B concentration and to determine the eligibility of a patient for BNCT, by calculating the lesion-to-normal (L/N) ratio of 18F-labeled BPA. The uptake in 11C-methionine-PET, which has been more extensively studied for cancer diagnoses, is shown to have a linear correlation with that of 18F-BPA-PET (Figure 3), indicating the potential application of 11C-methionine-PET for BNCT dose planning and candidate selection (11). Before neutron irradiation, boron compounds (BSH and/or BPA) are administered intravenously, and then blood samples are drawn serially after the intravenous injection of the boron agent to measure their level in the blood.
Neutron source for BNCT: from reactor to accelerator
The major issues in BNCT research concern the neutron sources, boron compounds, and clinical applications. BNCT research has been conducted for more than 60 years using nuclear research reactors. The first clinical studies for malignant brain tumors were performed at Brookhaven National Laboratory (BNL) and Massachusetts Institute of Technology (MIT) in 1950s and 1960s. In these early BNCT trials, low-energy thermal neutron beams were used for irradiation; however, because of shallow penetration, BNCT with thermal neutrons required craniotomy, to allow the neutrons to reach deeper regions of the brain.
In the 1990s, external beam BNCT using higher-energy (0.53-10 keV) epithermal neutrons was initiated using the Brookhaven Medical Research Reactor (BMRR) at BNL and a High Flux Reactor (HFR) at Petten, the Netherlands. This extended the therapeutic range deeper into the brain from 4 to 8 cm, and allowed the application of nonoperative external beam irradiation (12).
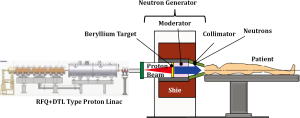
A typical research reactor for BNCT has only one irradiation port fixed in the side wall of the irradiation room, and this limits achieving desirable dose distribution compared to the current multiple field irradiation or conformation radiotherapy. The locations of research reactors usually require the transfer of the patient from a hospital, and this is unusually not possible until a few weeks after surgery. In Japan, the availability of machine time is limited by research projects, maintenance, and inspections. To resolve these nuclear reactor limitations, in-hospital accelerator-based neutron sources have been developed and are now providing neutron beams for clinical study of BNCT. The accelerator BNCT system consists of a proton accelerator, target, moderator, collimator, and irradiation room, and neutrons are provided by the reaction of the target material (Be, Li, etc.) and the accelerated protons (Figure 4). The first clinical trial of BNCT for brain tumors using the beryllium target accelerator system was initiated at KURRI in Japan in late 2012.
Boron compound and delivery system
A variety of boron delivery agents have been investigated to date, including amino acids, porphyrins, nanoparticles, polyamines, biochemical precursors, DNA-binding agents, sugars, antisense agents, peptides, proteins, monoclonal antibodies, and liposomes. However, there are only two boron delivery agents available for clinical BNCT trials for malignant glioma: 10B-enriched BPA and BSH (Figure 1). 10B constructs 20% of natural nonradioactive boron and has high efficiency in capturing thermal neutrons to generate boron neutron capture reaction, 10B(n, α) 7Li (13). Successful BNCT is dependent on the selective accumulation and absolute level of 10B atoms in tumor cells.
These boron delivery agents must be as safe as glucose, and drug administration of gram-order is commonly needed to achieve a high enough intracellular boron level to sterilize tumor cells. A boron delivery agent should be non-toxic at the clinically effective doses, achieve at least 10-30 µg 10B/g of tumor, have high tumor/brain and tumor/blood concentration ratios, and show rapid clearance from the blood circulation and normal tissues (but persist in the tumor). They should also be water soluble and chemically stable (14).
BPA has structural characteristics similar to those of a melanin precursor, and promising clinical results were shown in a pilot study of BNCT for skin melanoma (14). BPA is usually administered intravenously as a soluble fructose complex, BPA-F, at doses ranging from 250 to 900 mg BPA/kg. BPA can penetrate across the blood-brain barrier into the normal brain, and is actively transported through the tumor cell membrane due to the elevated rate of amino acid transport in proliferating cells. Although the uptake of BPA depends highly on individual tumors, high tumor-to-normal-BPA-uptake ratios (2.1-7.1) were demonstrated in an 18F-BPA-PET study of newly diagnosed GBMs (15).
BSH biodistribution studies have suggested that BSH is distributed through passive diffusion from the blood to tumor tissues via the disrupted blood-brain barrier. The boron concentration in the normal brain with an intact blood-brain barrier remains minimal, whereas the tumor 10B concentration is related to both the tumor vessel density and the blood 10B level. Tumor-to-blood boron concentration ratios ranging from 0.5 to 1.0 have been reported in human patients treated with BSH-mediated BNCT (12). Vascular irritation, fever, skin reaction (erythema), and peripheral vasoconstriction have been reported as probable adverse effects of BSH injection (10). Japanese clinical trials have used a combination of BPA and BSH based on experimental data which showed these different compounds accumulate in different subpopulations of tumor cells (12).
Clinical studies of BNCT
In a clinical trial using epithermal neutrons at the BNL in which 53 GBMs were irradiated to evaluate the safety and effectiveness of external beam BNCT (16,17), no major adverse events were found following the 2-h intravenous injection of BPA-F at a dose of 250 to 330 mg/kg. However, patients who received 330 mg/kg BPA showed precipitates in the urine. MST following one, two and three field (one fraction each) BNCT were 14.8, 12.1 and 11.9 mos, respectively. Two of the seven subjects received an average brain dose (ABD) of 8 Gy-Eq or above, using three fields, and had grade 3 CNS toxicity. An ABD of 6.2 Gy-Eq was associated with 50% incidence of somnolence. Other grade 3 radiation toxicity was ototoxicity (17,18).
In the clinical trial at Harvard/MIT (19), no adverse event was found in relation to the intravenous injection of 250 mg/kg over 1 h, 300 mg/kg over 1.5 h, and 350 mg/kg over 1.5 h. The tumors with volumes <60 cm3 and >60 cm3 were associated with a 19% and 67% incidence of developing grade 3 or higher toxicity, respectively. Experimental data suggest that a longer infusion time up to 6 hours may improve the homogeneity of boron accumulation in tumors in BPA-mediated BNCT (20,21). This method was applied to the phase II clinical trial at the Studsvik BNCT facility for 29 patients suffering from GBM, who received 900 mg/kg BPA-F in a 6-h infusion, where the average boron concentration in the blood was 24.7 µg/g (22,23). Four patients developed grade 3-4 toxic events including epileptic seizures, hematuria, thrombosis, and erythema. These events except for seizures may relate to BPA administration. The median progression free survival and median MST were 5.8 and 17.7 mos, respectively.
The Finnish phase I/II trial showed that the BPA dose level of 450 mg/kg was the optimal dose for further BNCT studies of newly diagnosed GBM (24,25). In that study, 290 mg/kg of BPA was infused over 2 h in the first 12 patients suffering from GBM using two fields, and the BPA dose to subsequent patients was escalated from 330 mg/kg (n=1) to 360 mg/kg (n=3), 400 mg/kg (n=3), 450 mg/kg (n=3), and 500 mg/kg (n=8). The maximum tolerated dose was reached at the BPA dose level of 500 mg/kg, where grade 3 (n=2) and grade 4 (n=1) CNS toxicity was found. Kankaanranta et al. (26) also reported a phase I dose escalation study for recurrent malignant glioma after initial treatment using X-ray fractionated radiotherapy at a dose of 50 to 61Gy, and they recommended up to 400 mg/kg L-BPA as a 2-h infusion. The MST values for the dose groups of 290, 330/360, 400, 450, and 500 mg BPA/kg were 13.4, 11.0, 16.9, 21.9 and 14.7 mos, respectively. The other studies’ protocol using long-term infusions showed that the median time from BNCT treatment to tumor progression was 5.8 mos, and the MST after BNCT was 14.2 mos (22,23).
The longer perfusion method was also employed in a trial at Osaka Medical College (700 mg/kg for 6 h) (15). Experimental data also suggest that the combination of BNCT and photon radiation leads to significant gains in survival (21). In the trial conducted at Osaka Medical College, the first 10 patients suffering from GBM were administered 100 mg/kg of BSH and 250 mg/kg of BPA in a 1-h infusion (protocol 1), and the latter 11 patients were administered 100 mg/kg of BSH and 700 mg/kg of BPA in a 6-h infusion (protocol 2). A 2 Gy daily fraction of X-ray irradiation was added in protocol 2 for a total dose of 20 to 30 Gy. The MST for all patients and for protocol 2 patients were 15.6 and 23.5 mos, respectively (15).
In a trial at the University of Tsukuba and Tokushima University at Japan Research Reactor No. 4 (JRR-4) of the Japan Atomic Energy Agency (JAEA) (10), the low dose (250 mg/kg) of BPA was administered over 1 h and 5 g BSH /kg was infused over 1 h in 8 patients with a single irradiation field. These patients received additional photon radiation defining the signal abnormality in T2-weighted MRI after the completion of BNCT. The MST and the time to progression were 27.1 and 11.9 mos, respectively. The 1-year and 2-year survival rates were 87.5% and 62.5%, respectively. This small number of patients showed the most favorable outcome with BNCT to date and treatment was well tolerated without severe acute or subacute adverse events. Four of 15 patients showed delayed radiation necrosis and median survival time of 4 patients including 1 alive patient was 43.4 mos (15.1-76.0). Although it is not certain whether the additional photon irradiation has a role in the clinical response to BNCT, the survival of the small cohort seemed to be favorable.
The clinical studies for newly diagnosed GBM revealed that the median time to progression varies from 6 to 12 mos and the MST varies from 12 to 27 mos after BNCT as an initial treatment (10). More clinical data are needed to confirm the effectiveness of this modality, although the existing results appear promising, and warrant further investigation. Future areas of research include clinical applications, the development of new boron delivery agents, and accelerator neutron sources.
Acknowledgments
Funding: This study was supported in part by the Grant-in-Aid for Society Collaboration from the Ministry of Education, Science and Culture, Japan (22591604).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.04.11). KT serves as an unpaid editorial board member of Translational Cancer Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006;7:392-401. [PubMed]
- Nimsky C, Ganslandt O, Buchfelder M, et al. Intraoperative visualization for resection of gliomas: the role of functional neuronavigation and intraoperative 1.5 T MRI. Neurol Res 2006;28:482-7. [PubMed]
- Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 2002;359:1011-8. [PubMed]
- Fadul CE, Wen PY, Kim L, et al. Cytotoxic chemotherapeutic management of newly diagnosed glioblastoma multiforme. J Neurooncol 2008;89:339-57. [PubMed]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [PubMed]
- Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 2003;5:79-88. [PubMed]
- Valtonen S, Timonen U, Toivanen P, et al. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery 1997;41:44-8; discussion 48-9. [PubMed]
- Tanaka M, Ino Y, Nakagawa K, et al. High-dose conformal radiotherapy for supratentorial malignant glioma: a historical comparison. Lancet Oncol 2005;6:953-60. [PubMed]
- Fitzek MM, Thornton AF, Rabinov JD, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg 1999;91:251-60. [PubMed]
- Yamamoto T, Nakai K, Kageji T, et al. Boron neutron capture therapy for newly diagnosed glioblastoma. Radiother Oncol 2009;91:80-4. [PubMed]
- Nariai T, Ishiwata K, Kimura Y, et al. PET pharmacokinetic analysis to estimate boron concentration in tumor and brain as a guide to plan BNCT for malignant cerebral glioma. Appl Radiat Isot 2009;67:S348-50. [PubMed]
- Yamamoto T, Nakai K, Matsumura A. Boron neutron capture therapy for glioblastoma. Cancer Lett 2008;262:143-52. [PubMed]
- Nielsen FH. Micronutrients in parenteral nutrition: boron, silicon, and fluoride. Gastroenterology 2009;137:S55-60. [PubMed]
- Coderre JA, Turcotte JC, Riley KJ, et al. Boron neutron capture therapy: cellular targeting of high linear energy transfer radiation. Technol Cancer Res Treat 2003;2:355-75. [PubMed]
- Kawabata S, Miyatake S, Kuroiwa T, et al. Boron neutron capture therapy for newly diagnosed glioblastoma. J Radiat Res 2009;50:51-60. [PubMed]
- Chanana AD, Capala J, Chadha M, et al. Boron neutron capture therapy for glioblastoma multiforme: interim results from the phase I/II dose-escalation studies Neurosurgery 1999;44:1182-92; discussion 1192-3. [PubMed]
- Diaz AZ. Assessment of the results from the phase I/II boron neutron capture therapy trials at the Brookhaven National Laboratory from a clinician’s point of view. J Neurooncol 2003;62:101-9. [PubMed]
- Coderre JA, Hopewell JW, Turcotte JC, et al. Tolerance of normal human brain to boron neutron capture therapy. Appl Radiat Isot 2004;61:1083-7. [PubMed]
- Busse PM, Harling OK, Palmer MR, et al. A critical examination of the results from the Harvard-MIT NCT program phase I clinical trial of neutron capture therapy for intracranial disease. J Neurooncol 2003;62:111-21. [PubMed]
- Yoshida F, Matsumura A, Shibata Y, et al. Cell cycle dependence of boron uptake from two boron compounds used for clinical neutron capture therapy. Cancer Lett 2002;187:135-41. [PubMed]
- Barth RF, Coderre JA, Vicente MG, et al. Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res 2005;11:3987-4002. [PubMed]
- Henriksson R, Capala J, Michanek A, et al. Boron neutron capture therapy (BNCT) for glioblastoma multiforme: a phase II study evaluating a prolonged high-dose of boronophenylalanine (BPA). Radiother Oncol 2008;88:183-91. [PubMed]
- Sköld K. Boron Neutron Capture Therapy for glioblastoma multiforme: advantage of prolonged infusion of BPA-f. Acta Neurol Scand 2010;122:58-62. [PubMed]
- Joensuu H, Kankaanranta L, Seppälä T, et al. Boron neutron capture therapy of brain tumors: clinical trials at the finnish facility using boronophenylalanine. J Neurooncol 2003;62:123-34. [PubMed]
- Kankaanranta L, Koivunoro H, Kortesniemi M, et al. BPA-based BNCT in the treatment of glioblastoma multiforme. A dose escalation study. In: Zonta A, Altieri S, Roveda L, et al. eds. Proceedings of the 13th international congress of neutron capture therapy, EANA, Rome, 2008,30.
- Kankaanranta L, Seppälä T, Koivunoro H, et al. L-boronophenylalanine-mediated boron neutron capture therapy for malignant glioma progressing after external beam radiation therapy: a Phase I study. Int J Radiat Oncol Biol Phys 2011;80:369-76. [PubMed]


