
When tumors are (co-)opting to resist anti-angiogenic treatment
The role of angiogenesis, i.e., the growth of new blood vessels, in tumor biology revolutionized targeted cancer therapy when it was introduced approximately 10–15 years ago (1). Today, however, we know that anti-angiogenic treatment with either antibodies or small molecular inhibitors of the vascular endothelial growth factor (VEGF)-A—VEGF receptor (VEGFR)-2 axis are less effective in most malignant indications than initially expected (2). Such agents are, however, very effective at blocking tumor growth in most pre-clinical models, due to their potent effects on regressing the tumor vasculature leading to tumor hypoxia and necrosis. Recent scrutiny of the differences between such pre-clinical models and the clinical reality have lifted up a number of issues with the most commonly used mouse tumor models that could be important to understand the discrepancies observed in the effects of anti-angiogenic drugs, including age, genetic variability, the tissue in which the tumor grows etc. (1). Another emerging difference of potentially critical importance is the circadian rhythmicity of the host which is generally non-perturbed in tumor baring mice but often disrupted in cancer patients (3-5). This commentary is dedicated to discussing recent clinical and pre-clinical evidence in support of a mechanism that previously has not gained much attention but may be critical for our understanding of how anti-angiogenic resistance develops in cancer. That mechanism is vascular co-option.
Vascular co-option is the process of taking over and incorporating or “hijacking” the existing vasculature as the blood supply for the tumor (6,7). This can be envisaged to happen in two ways. Firstly, tumors, especially those rich in myofibroblasts, may pull adjacent blood vessels into the tumor. Such a process was recently shown to be important for initial vascularization of granulation tissue during wound healing and in the injured mouse cornea (8) but whether such a process could also be involved in tumor vascularization has not yet been investigated. More commonly, co-option is considered to occur by invasion of the tumor into the adjacent tissue, where the tumor cells migrate and grow predominantly in the perivascular environment (6,7) (Figure 1).
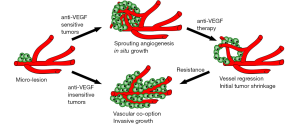
The role of vascular co-option during growth of micro-metastatic lesions was recently convincingly demonstrated using highly sophisticated intra-vital microscopy to study the early events of brain metastasis over the course of several months in living mice (9). In this study, several critical steps involved in the metastatic process including the extended luminal arrest of cancer cells prior to extravasation and the tight coupling of tumor cells to the abluminal vessel wall for days or weeks following extravasation, was identified. Interestingly, melanoma brain metastases primarily grew along and thus co-opted the brain vasculature and rarely induced sprouting and angiogenic growth of the vessels as they expanded to macro-lesions. This process of vascular co-option was independent of VEGF-A, as treatment with the VEGF-A neutralizing antibody bevacizumab did not inhibit melanoma brain metastatic growth. On the other hand, lung cancer cells proliferated in situ, recruited new vessels to the tumor by angiogenic sprouting and did not co-opt existing vessels to a significant extent. This angiogenic induction depended on VEGF-A signaling and as such, lung cancer brain metastases were significantly inhibited by bevacizumab treatment. Following a time of inhibited growth, however, the lung cancer metastatic lesions switched to a migratory and invasive phenotype, similar to the melanoma lesions, co-opted the host vasculature and grew in spite of continued bevacizumab treatment (9) (illustrated in Figure 1).
A variation of this study was executed by Zhao et al., using a zebrafish xenograft model in which tumors were implanted into the perivitelline space or the pericardial membrane, tissues of high or low intrinsic vascular density respectively, and their growth patterns were examined by intravital microscopy over time (10). In contrast to the general belief that vascular co-option is more frequently observed in tissues with a high vascular density (11), this study found that tumor cells, mainly co-opted the host vasculature in the poorly vascularized pericardial membrane, whereas when implanted in the densely vascularized perivitelline space they preferred to grow by angiogenic recruitment of new vessels into the tumor mass (10). Lim et al. have also recently studied the role of vascular density on tumor cell co-option investigating the growth of various different tumor cells including breast cancer, melanoma and fibrosarcoma in adipose tissues exhibiting varying vascular densities (12). The growth rate of tumors implanted in brown adipose tissue was found to be much faster than that of tumors implanted in white adipose tissue, which in turn was much faster than that of tumors implanted sub-cutaneously. These growth rates directly correlated with vascular density of the tissue, the invasiveness of the tumors and thus with the degree of vascular co-option (12). This is the first time vascular co-option has been demonstrated in adipose tissue, leading to the intriguing hypothesis that tumors growing in such environments may be less sensitive to anti-VEGF treatment. This interesting issue warrants further study.
Interestingly, the choice of co-option versus angiogenesis may at least in part depend on the tumor size, as small tumors were more likely to co-opt the existing vasculature, whereas larger masses more readily induced angiogenesis, in the zebrafish tumor xenograft model (10). Indeed, in this model system the two processes were mutually exclusive, when tumors had grown to a size sufficient for induction of angiogenesis, which for VEGF-A expressing lung cancer cells were significantly smaller than for the non-VEGF-A expressing melanoma cells, they no longer used co-option as a mechanism for vascularization (12). This is in line with the observations of Runge et al. that in an inducible model of hepatocellular carcinoma (HCC) found that at initial stages of tumorigenesis, malignant cells grew by vascular co-option but as the lesions had grown to a critical size they switched to an angiogenic phenotype at which point the growth could be inhibited by anti-VEGF therapy (13).
In agreement with the findings of Kienast et al., acquired resistance and tumor regrowth during sorafenib treatment of HCC was recently found to depend on a switch to a vascular co-option phenotype rather than rebound sprouting angiogenesis (14). In this model, orthotropically implanted and non-treated HCC lesions were uninvasive, highly hemorrhagic and large. Sorafenib (an inhibitor of VEGF-signaling) treatment, while effectively reducing tumor vascular density, size and inducing tumor necrosis, however, also led to a more invasive phenotype as the tumors developed resistance to the treatment. Such an invasive phenotype of the resistant tumors was associated with increased levels of VEGF-A and Osteopontin, the levels of which, surprisingly correlated negatively with intratumoral vessel density (14). Both sorafenib sensitive and resistant tumors exhibited many tumor cells that had undergone EMT, indeed, resistant and histologically more invasive tumors seemed to have a lower overall percentage of mesenchymal-like tumor cells, compared to the sensitive, dormant tumors. Upon cessation of sorafenib treatment, however, rapid rebound angiogenesis, partial MET and accelerated tumor regrowth was observed (14). The effects of stopping anti-VEGF treatment were also recently studied by Yang et al. In this study, rebound angiogenesis was found to be associated with significant growth of sinusoidal fenestrae (holes in the vessels) leading to increased leakage and either tumor cell extravasation and liver metastasis formation or intravasation by HCC cells and metastasis to the lung (15). As such, anti-VEGF treatment, especially if not administered continuously, may be associated with increased invasiveness, hematogenous dissemination and metastasis.
While the importance of vascular co-option in tumor biology has mostly been studied in pre-clinical models, a recent study by Frentzas et al., convincingly demonstrate that this is a highly clinically relevant issue which affect the anti-VEGF sensitivity of the tumors and may be used as a histological biomarker (16). In this study, patients having liver metastases (from colorectal or breast cancer origin) which exhibited a predominantly invasive phenotype with a high degree of vascular op-option (aka displacement histopathologic growth pattern) had significantly poorer response to neoadjuvant treatment including bevacizumab compared to patients with predominantly non-invasive (aka desmoplastic) phenotypes (16). Interestingly, these authors found that tumor cell migration through Arp2/3-induced actin cytoskeletal rearrangements were critical for successful vascular co-option. Reducing Arp2/3 expression in tumor cells led to a markedly reduced invasiveness and increased sensitivity to anti-VEGF treatment compared to Arp2/3 competent tumors in pre-clinical liver cancer models (16).
Taken together, these and other recent insights into vascular co-option in cancer strongly suggest that vascular co-option is commonly seen in both pre-clinical and clinical cancer studies and associated with invasive phenotypes and poor response/resistance to anti-VEGF therapy. As such, future studies should focus on elucidating the mechanisms underlying vascular co-option as this is an area still very much in its infancy, but with an enormous potential for paving the way for novel anti-cancer therapies.
Acknowledgments
Dr. Jensen’s lab is supported by the Swedish Society for Medical Research (SSMF), The Ollie and Elof Ericsson’s Foundation, and the Swedish Research Council (VR).
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Bo Zhai (Department of Hepatobiliary Surgery, The Fourth Hospital of Harbin Medical University, Harbin, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.35). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cao Y, Arbiser J, D'Amato RJ, et al. Forty-year journey of angiogenesis translational research. Sci Transl Med 2011;3:114rv3 [Crossref] [PubMed]
- Ribatti D. Tumor refractoriness to anti-VEGF therapy. Oncotarget 2016;7:46668-77. [PubMed]
- Jensen LD. The circadian clock and hypoxia in tumor cell de-differentiation and metastasis. Biochim Biophys Acta 2015;1850:1633-41.
- Jensen LD, Gyllenhaal C, Block K. Circadian angiogenesis. Biomol Concepts 2014;5:245-56. [Crossref] [PubMed]
- Jensen LD, Cao Z, Nakamura M, et al. Opposing effects of circadian clock genes bmal1 and period2 in regulation of VEGF-dependent angiogenesis in developing zebrafish. Cell Rep 2012;2:231-41. [Crossref] [PubMed]
- Qian CN. Hijacking the vasculature in ccRCC--co-option, remodelling and angiogenesis. Nat Rev Urol 2013;10:300-4. [Crossref] [PubMed]
- Ribatti D, Vacca A, Dammacco F. New non-angiogenesis dependent pathways for tumour growth. Eur J Cancer 2003;39:1835-41. [Crossref] [PubMed]
- Kilarski WW, Samolov B, Petersson L, et al. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat Med 2009;15:657-64. [Crossref] [PubMed]
- Kienast Y, von Baumgarten L, Fuhrmann M, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 2010;16:116-22. [Crossref] [PubMed]
- Zhao C, Yang H, Shi H, et al. Distinct contributions of angiogenesis and vascular co-option during the initiation of primary microtumors and micrometastases. Carcinogenesis 2011;32:1143-50. [Crossref] [PubMed]
- Coelho AL, Gomes MP, Catarino RJ, et al. Angiogenesis in NSCLC: is vessel co-option the trunk that sustains the branches? Oncotarget 2016; [Epub ahead of print]. [PubMed]
- Lim S, Hosaka K, Nakamura M, et al. Co-option of pre-existing vascular beds in adipose tissue controls tumor growth rates and angiogenesis. Oncotarget 2016;7):38282-91.
- Runge A, Hu J, Wieland M, et al. An inducible hepatocellular carcinoma model for preclinical evaluation of antiangiogenic therapy in adult mice. Cancer Res 2014;74:4157-69. [Crossref] [PubMed]
- Kuczynski EA, Yin M, Bar-Zion A, et al. Co-option of Liver Vessels and Not Sprouting Angiogenesis Drives Acquired Sorafenib Resistance in Hepatocellular Carcinoma. J Natl Cancer Inst 2016;108:djw030 [Crossref] [PubMed]
- Yang Y, Zhang Y, Iwamoto H, et al. Discontinuation of anti-VEGF cancer therapy promotes metastasis through a liver revascularization mechanism. Nat Commun 2016;7:12680. [Crossref] [PubMed]
- Frentzas S, Simoneau E, Bridgeman VL, et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med 2016;22:1294-302. [Crossref] [PubMed]


