
miR-542-3p suppresses colorectal cancer progression through targeting survivin
Introduction
Colorectal cancer (CRC) ranks as the third most common cancer after lung cancer and breast cancer worldwide, with its morbidity and mortality still increasing in recent years (1,2). Although there are significant advances in the treatment of CRC patients, the overall survival (OS) rate of CRC patients has not been improved markedly. Therefore, identification of important regulators during occurrence and progression of CRC and non-invasive biomarkers for the early detection of CRC would be crucial for the development of effective interventions.
Numerous miRNAs act as either tumor suppressors or tumor promoters, and the aberrant miRNA expression is directly associated with cancer occurrence, progression, metastasis, tumor relapse and drug resistance in a wide variety of human malignancies (3). Recent studies have shown that miR-542-3p suppresses tumor cell growth, invasion, and angiogenesis in several tumors (4-6) by targeting genes which are critically associated with cell proliferation, invasion and angiogenesis. Survivin is an important member of the inhibitor of apoptosis (IAP) family and it suppresses apoptosis by interacting with caspases via baculovirus IAP repeat domains to inhibit caspase activation (7). Dysregulation of survivin is a key feature of many cancers, including CRC. Previous studies have showed that survivin could inhibit apoptosis, enhance cell proliferation in CRC and was associated with poor prognosis of the patients (8,9).
Here, we sought to investigate the expression levels and functional implications of miR-542-3p in CRC. We also identified survivin as a direct target of miR-542-3p in CRC. Our study showed the potential therapeutic and diagnostic value of miR-542-3p in CRC patients.
Methods
Patients and samples
Fresh colorectal tumor and matched normal colorectal mucosa specimens from 65 patients who were diagnosed and underwent surgery in Peking University People’s Hospital between 2010 and 2013 were included in this study. None of the patients received chemotherapy or radiation therapy before surgery. All patients were followed up postoperatively for at least 3 years. In the 65 cases of CRC, patients with miR-542-3p levels lower or higher than the median level were defined as low miR-542-3p expression group and high miR-542-3p expression group, respectively. Plasma samples from another 52 CRC patients who were diagnosed and underwent surgery in Peking University People’s Hospital between 2014 and 2015 were collected just before surgery and initiation of any oncological treatment. Blood samples from 30 age-matched healthy donors were collected as controls. The study was approved by the Institutional Review Boards of Peking University People’s Hospital (ethical approval number: 2010-58), and written informed consent was obtained from each subject.
RNA extraction and real-time PCR
For cell lines and tissue samples, total RNA was extracted using Trizol (Invitrogen) according to the manufacturer’s instructions. For the plasma, the miRNAs were extracted using mirVanaTM PARISTM microRNA extraction kit (ABI) according to the manufacturer’s instructions. For survivin quantification, actin was used as an endogenous control. The qPCR primers were as follows. Survivin: TCCGCAGTTTCCTCAAATTC (forward) and TTGCGCTTTCCTTTCTGTC (reverse); actin: CCTTGCACATGCCGGAG (forward) and GCACAGAGCCTCGCCTT (reverse). For miR-542-3p quantification, U6 small nuclear RNA was used as an internal control. TaqMan microRNA assays for miR-542-3p (001284) and U6 snRNA (001973) were from Applied Biosystems. All reactions were performed in triplicate. The fold change for each gene relative to the control group was calculated using the 2−ΔΔCt method.
Western Blot
Proteins were extracted from cell lines or tissues using sodium dodecyl sulfate lysis buffer (2% sodium dodecyl sulfate, 10% glycerol, 0.1 mM dithiothreitol, and 0.2 M Tris-HCl, pH 6.8). Protein samples were resolved by SDS–PAGE and analyzed by immunoblots. Survivin antibody was from abcam (ab137650).
Cell culture and transfection
Colon cancer cell lines LoVo, HCT116, HT29, RKO, SW480, and SW620 were obtained from American Type Culture Collection (ATCC) and subcultured and preserved by our lab. All the cells were maintained in Dulbecco’s Modified Essential Medium (DMEM) with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 mg/mL streptomycin. Cells were cultured in a humidified atmosphere with 5% CO2 at 37 °C. The miR-542-3p mimics, mimics control, miR-542-3p inhibitor and inhibitor control were from Ambion. For transient cell transfection, cells were plated in 12-well plates and Lipofectamine 3000 (Invitrogen) was used for transfection. For stable transfection, lentiviral vectors (GenePharma, Shanghai, China) containing miR-542-3p (LV-miR-542-3p) or negative control sequences (LV-NC) were applied, and the cells were cultured with antibiotic selection medium (puromycin 5 µg/mL) for 4–5 days.
Luciferase activity assay
To construct the 3'-UTR luciferase vectors of survivin, the wild type or mutated 3'-UTR sequence of the human survivin containing the putative target site for miR-542-3p was amplified by PCR and cloned into pMIR-report vector (Promega). For luciferase reporter assays, SW480 cells were transiently transfected with pMIR-report containing survivin 3'-UTR and miR-542-3p mimics. After 48 h, reporter activity was measured using the dual-luciferase assay system (Promega). Renilla luciferase activity was used to normalize for transfection efficiency.
Cell proliferation and colony formation assay
Cell proliferation was tested by CCK8 assay according to the manufacturer’s instructions. The absorbance was measured at 450 nm using a microplate reader (Bio-Rad). The CCK8 assay was repeated for 3 times with 5 replicates. The colony formation assay was performed as follows: after transfection, cells were seeded into each well of a 6-well plate on day 0 then incubated for another 12 d. The wells were then washed with PBS, fixed with 4% paraformaldehyde, and stained with 0.1% crystal violet. The colonies were counted and photographed.
Cell invasion assay
Cell invasion assay was performed using Corning Polycarbonate Membrane Insertin transwell chamber (Product #3422, Corning Costar Corp, Cambridge, MA, USA). Cells (1×105) were suspended in 200 uL serum-free medium and seeded into the upper chamber of a transwell inserted with an 8-µm pore size membrane and coated with matrigel (Sigma). DMEM containing 20% FBS was placed in the lower chamber as a chemoattractant. After incubating for 48 h, the non-invading cells were removed with cotton swabs. Invasive cells at the bottom of the membrane were stained with 0.1% crystal violet and were counted under microscopic observation.
Cell cycle and apoptosis analysis
For cell cycle analysis, BD Cycletest™ Plus DNA Kit was used. The transfected cells were harvested, washed with cold PBS, then fixed in cold 75% ethanol at 4 °C. After staining with propidium iodide (PI) solution for 30 min in dark, cell cycle analysis was performed by fluorescence activated cell sorting.
For cell apoptosis analysis, cells were trypsinized 72 h after transfection and stained with BD FITC Annexin V Apoptosis Detection Kit I. All cells were detected by flow cytometry (BD Biosciences).
Xenograft animal model
Four-week-old female athymic BALB/c were used for in vivo distant metastasis assay. miR-542-3p stable over-expression RKO cells (RKO-LV-miR-542-3p) and control cells (RKO-LV-NC) were injected into the inferior pole of the spleen of the mice under anesthesia. Five weeks later, the mice were sacrificed, and the liver and spleens were removed. Hematoxylin and eosin (HE) staining was performed to exam the formed tumors.
Statistical analysis
Statistical analysis was performed by SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). All in vitro experiments were performed in triplicate and repeated for 3 times. Two-tailed Student’s t test was used to compare mean values. Pearson correlation coefficient was used for correlation analysis. The survival curves were estimated by Kaplan-Meier analysis, and P values were calculated by log rank test. Statistically significant differences were defined as P<0.05. For all, *P<0.05, **P<0.01, ***P<0.001.
Results
miR-542-3p was decreased in tumor tissues of CRC patients
We firstly examined the expression of miR-542-3p in 6 CRC cell lines, including LoVo, RKO, SW480, SW620, HT-29, HCT116, and the relative expression levels are shown in Figure 1A. We noticed that miR-542-3p expression was lower in LoVo cell line which was derived from a metastatic site of CRC. While in cell lines derived from primary tumor site, such as HT-29 and HCT116, its expression was relatively higher. Especially, in cell lines derived from the same patient, its expression was lower in metastatic site-derived SW620 than the primary tumor site-derived SW480.
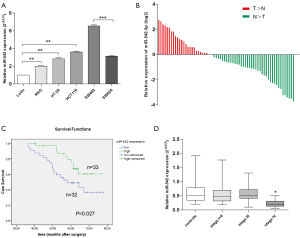
Then, the expression of miR-542-3p in 65 CRC cancerous tissues and paired normal tissues was determined using real-time PCR. A decrease of miR-542-3p expression in tumor tissues was seen in 63.08% (41/65) of the patients. miR-542-3p expression was significantly down-regulated in tumor tissues compared with the paired adjacent normal tissues (Wilcoxon test, P=0.006; Figure 1B). After stratification of the patients according to their TNM stages, we found there was a decreased trend of miR-542-3p expression with the stages of the patients advanced, and the relative expression level of miR-542-3p were (set stage I as 1): 1, 0.89, 0.67, and 0.34. These data indicate that abnormal miR-542-3p expression may be related to CRC pathogenesis.
Decreased miR-542-3p indicated poor prognosis of CRC patients
To assess the correlation of miR-542-3p expression with clinicopathological factors, the expression level of miR-542-3p in the above 65 CRC cancerous tissues were categorized into high (n=33) and low (n=32) groups according to the median expression level. As shown in Table 1, there was no significant correlation between the expression level of miR-542-3p and gender, age, differentiation, tumor size, and depth of invasion of the patients. However, the low miR-542-3p expression group showed a more lymphovascular invasion (P=0.008), distant metastasis (P=0.006), and a later tumor stage (P=0.034) than the high expression group.
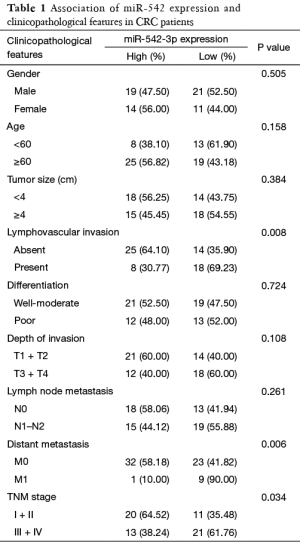
Full table
Then the prognostic implication of miR-542-3p in the above 65 CRC patients was evaluated. The overall median survival time of all the patients was 60.17 months. During the follow-up duration, 28 patients died of CRC. Kaplan-Meier analysis showed that patients with high expression level of miR-542-3p had significantly longer OS time than those with low expression level (P=0.027, Figure 1C). Then, to test whether miR-542-3p expression was an independent prognostic factor for CRC patients, multivariate COX regression analysis was performed (Table 2), in which those parameters associated with prognosis in the univariate survival analysis, including miR-542-3p expression level, lymphovascular invasion, and TNM stage, were included. We found that lymphovascular invasion, tumor TNM stage were independent prognostic factors, while miR-542-3p was not (P=0.013, <0.001, and 0.763, respectively).
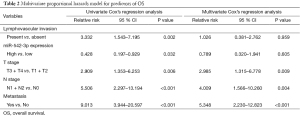
Full table
Plasma miR-542-3p were decreased in stage IV CRC patients
As the peripheral blood is easily accessible and it has been seen as a good source for cancer biomarkers, we sought to investigate the miR-542-3p levels in the plasma from another 52 CRC patients and 30 normal controls. In our analysis, stage I and stage II cases were grouped together because of limited stage I cases. We did not detect a significantly difference between CRC patients and normal controls. While after stratification by patients’ clinical stages, miR-542-3p was significantly down-regulated in stage IV patients than stage I–II, stage III or normal controls, respectively (Figure 1D).
MiR-542-3p inhibits the aggressive phenotypes of CRC cells in vitro and in vivo
To exam the functional roles of miR-542-3p on the malignant phenotypes of CRC cells, in vitro and in vivo experiments were conducted. We firstly transfected SW480 and LoVo cells with miR-542-3p mimics and inhibitors. Real-time PCR results showed that the mimics could increase miR-542-3p expression when compared with mimics NC, while the inhibitor suppressed miR-542-3p expression in comparison with inhibitor NC (Figure 2A). Then, CCK8 assay showed that transfection with miR-542-3p mimics inhibited proliferation of SW480 and LoVo cells when compared with the mimics control group (Figure 2B). Plate colony formation assay also showed that transfection of miR-542-3p mimics significantly reduced the colony formation of SW480 and LoVo cells (Figure 2C). Transwell migration and invasion assays showed that miR-542-3p could inhibit the migration and invasion abilities of SW480 cells (Figure 2D).
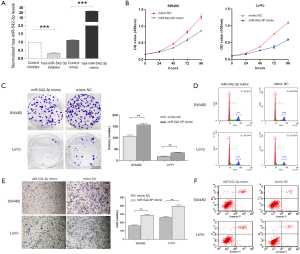
We further determined the effect of miR-542-3p on cell cycle and cell apoptosis. Flow cytometry analysis revealed that miR-542-3p over-expression resulted in G1-phase arrest of SW480 cells (from 55.33% to 60.23%, P=0.001) compared with the mimics control group. Consistently, miR-542-3p over-expression resulted in significant G1 arrest of LoVo cells (from 56.52% to 63.89%, P<0.001) (Figure 2E). In apoptosis assay, compared to the mimics control group, transfection of miR-542-3p increased the cellular apoptosis in SW480 (8.21% vs. 4.08%, P<0.001) and LoVo cells (8.46% vs. 4.32%, P<0.001, Figure 2F).
Distant metastasis is one of the most important phenotypes of malignant cancer cells, so we finally examined the impact of miR-542-3p on metastasis ability in vivo through a liver metastasis model on nude mice. Cells transfected with negative control lentivirus formed primary tumor in spleen in all four mice, with three of them suffered liver metastasis (detected grossly or microscopically), while cells transfected with miR-542-3p form tumors in spleens of two mice, and only one of them formed liver metastatic lesions (Figure 3A). The spleens and livers were excised and underwent HE staining (Figure 3B). These results strongly support the implication that miR-542-3p exerts suppressive effect in CRC distant metastasis.

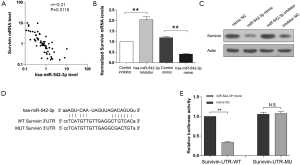
Survivin is a target of miR-542-3p in CRC
As survivin has been reported to be a target of miR-542-3p in some other cancers, we wondered whether miR-542-3p could also regulate survivin in CRC. Firstly, to investigate the relationship between miR-542-3p and survivin in CRC, correlation analysis between their expression levels was performed. Real-time PCR results showed that the expression of survivin was negatively correlated with the expression of miR-542-3p (r=–0.31, P=0.01, Figure 4A). Then, to validate that survivin is a target of miR-542-3p, we transfected miR-542-3p mimics or inhibitor into the colon adenocarcinoma cell line SW480. Results of real-time PCR (Figure 4B) and Western blot (Figure 4C) showed that miR-542-3p inhibitor could increase the mRNA and protein levels of survivin while miR-542-3p mimics could reduce survivin expression. To explore the potential effects of miR-542-3p on the 3'-UTR of survivin, we cloned the wild type and mutated 3'-UTR of survivin into a luciferase reporter vector pMIR (Figure 4D). In SW480 cells transfected with survivin luciferase reporter vector, miR-542-3p inhibitor could increase the luciferase activity while miR-542-3p mimics could reduce the luciferase activity (Figure 2E). Once the predicted binding sites in the 3'-UTR of survivin were mutated, the effects of miR-542-3p inhibitor or mimics were abolished, indicating that miR-542-3p could directly bind to the 3'-UTR of survivin (Figure 4E).
Discussion
We found in this study that expression of miR-542-3p in cancerous tissues was decreased in 65 CRC patients than paired normal mucosa, which was in accordance with results in bladder cancer (10), astrocytoma (5), and gastric cancer (11). The mechanism of miR-542-3p down regulation in CRC is unknown by far. A recent study shows that several transcription factors (Ap-2r, CEBPB, ELF-1, POU2F1, STAT3 and ZNF263) might bind to the promoter region of pri-miR-542 in breast cancer (12). It would be of great interest to investigate the potential roles of these transcription factors in CRC and whether they are responsible for the miR-542-3p down regulation in CRC. We also found that the expression of miR-542-3p was significantly associated to lymphovascular invasion, distant metastasis and tumor stage, and a higher miR-542-3p expression was predictive of a longer OS of the patients. Previously, miR-542-3p was found to be associated with advanced tumor stages and presence of tumor recurrence in bladder cancer (10). While in neuroblastoma patients, it was proved to be correlated with event-free survival of (13). As to CRC, Takeyama and colleagues showed that miR-542-3p expression was higher in patients with liver metastasis than those without liver metastasis (14). However, they detected no expression difference between 16 normal mucosa and 16 cancer tissues, and no effect of miR-542-3p on disease free survival (DFS) or OS of the patients (14). This discrepancy may be due to the differences in clinicopathological features of the patients, or population-specific factors such as different genetic backgrounds of the studied cohorts.
In accordance with its prognostic role, both our in vitro and in vivo experiments showed that miR-542-3p could inhibit the aggressive phenotypes of CRC cell lines, including proliferation, migration and invasion, colony formation, cell cycle, apoptosis, as well as in vivo liver metastasis. This is in consistent with a previous study, in which only in vitro experiments were included. In addition of CRC, miR-542-3p have also been proved to be a tumor suppressor in other cancers, including bladder cancer (10), melanoma (15), and gastric cancer (11).
Among the potential targets of miR-542-3p, survivin has been reported to be very crucial during the progression of CRC (16-19). While the mechanism of survivin over-expression in CRC is unclear and new insights for its regulatory mechanism in CRC might be beneficial for the survivin-based therapies. Here, we show evidence that survivin is a direct target of miR-542-3p in CRC. Our results are consistent and fully supported by results from other types of cancer. In lung carcinoma cell line A549, cervix adenocarcinoma cell line HeLa, breast adenocarcinoma cell line MCF-7, miR-542-3p could reduce survivin expression, inhibit cell proliferation and colony formation, and lead to G1 and G2/M arrest (20). Similarly, miR-542-3p exerts its tumor suppressive function in neuroblastoma by inhibiting survivin expression, decreasing cell proliferation and inducing apoptosis (13). Taken together, these results demonstrate a reproducible and important role for miR-542-3p in CRC by targeting survivin.
Many studies have demonstrated that circulating nucleic acids in plasma are potential biomarkers with non-invasiveness and low cost for cancer detection. Particularly, miRNAs were demonstrated to be remarkably stable in plasma (21). One previous study has shown that serum miR-542-3p was significantly decreased in patients with cervical squamous cell carcinoma than the normal controls (22). In this study, we found that although the plasma miR-542-3p levels in CRC patients and normal controls were not statistically different, it was down-regulated in stage IV CRC patients when compared with normal controls and stage I–II and stage III patients. This suggested that monitoring miR-542-3p level in the plasma might be helpful for identify those patients who have undergone a distant metastasis. What should be pointed out is that although we have detected a decreased trend of miR-542-3p expression in tissues along with increased CRC stages, this was not the case for plasma. We presume that miR-542-3p in the plasma might come from many organs and cell types besides tumor tissues, and the relation between miR-542-3p and tumor behavior may be diluted in stage I–III patients by the diverse sources of plasma miR-542-3p.
Conclusions
In conclusion, our study showed that miR-542-3p expression is decreased in CRC tissues than paired normal mucosa, and it is associated with lymphovascular invasion, distant metastasis and later tumor stages of the patients. Circulating miR-542-3p is decreased in patients with an advanced stage. miR-542-3p could inhibit the aggressive phenotypes of CRC cell line in vitro and in vivo, while survivin is a direct target of miR-542-3p in CRC. Further investigation with a larger cohort is required to validate these observations.
Acknowledgments
Funding: This study was funded by National Natural Science funding (Nos. 81372290, 81572379, 81572383).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.10). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Boards of Peking University People’s Hospital (No. 2010-58) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Palanichamy JK, Rao DS. miRNA dysregulation in cancer: towards a mechanistic understanding. Front Genet 2014;5:54. [Crossref] [PubMed]
- Kureel J, Dixit M, Tyagi AM, et al. miR-542-3p suppresses osteoblast cell proliferation and differentiation, targets BMP-7 signaling and inhibits bone formation. Cell Death Dis 2014;5:e1050 [Crossref] [PubMed]
- Cai J, Zhao J, Zhang N, et al. MicroRNA-542-3p Suppresses Tumor Cell Invasion via Targeting AKT Pathway in Human Astrocytoma. J Biol Chem 2015;290:24678-88. [Crossref] [PubMed]
- He T, Qi F, Jia L, et al. MicroRNA-542-3p inhibits tumour angiogenesis by targeting angiopoietin-2. J Pathol 2014;232:499-508. [Crossref] [PubMed]
- Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol 2002;3:401-10. [Crossref] [PubMed]
- Goossens-Beumer IJ, Zeestraten EC, Benard A, et al. Clinical prognostic value of combined analysis of Aldh1, Survivin, and EpCAM expression in colorectal cancer. Br J Cancer 2014;110:2935-44. [Crossref] [PubMed]
- Kim PJ, Plescia J, Clevers H, et al. Survivin and molecular pathogenesis of colorectal cancer. Lancet 2003;362:205-9. [Crossref] [PubMed]
- Zhang J, Wang S, Han F, et al. MicroRNA-542-3p suppresses cellular proliferation of bladder cancer cells through post-transcriptionally regulating survivin. Gene 2016;579:146-52. [Crossref] [PubMed]
- Shen X, Si Y, Yang Z, et al. MicroRNA-542-3p suppresses cell growth of gastric cancer cells via targeting oncogene astrocyte-elevated gene-1. Med Oncol 2015;32:361. [Crossref] [PubMed]
- He T, Qi F, Jia L, et al. Tumor cell-secreted angiogenin induces angiogenic activity of endothelial cells by suppressing miR-542-3p. Cancer Lett 2015;368:115-25. [Crossref] [PubMed]
- Althoff K, Lindner S, Odersky A, et al. miR-542-3p exerts tumor suppressive functions in neuroblastoma by downregulating Survivin. Int J Cancer 2015;136:1308-20. [Crossref] [PubMed]
- Takeyama H, Yamamoto H, Yamashita S, et al. Decreased miR-340 expression in bone marrow is associated with liver metastasis of colorectal cancer. Mol Cancer Ther 2014;13:976-85. [Crossref] [PubMed]
- Rang Z, Yang G, Wang YW, et al. miR-542-3p suppresses invasion and metastasis by targeting the proto-oncogene serine/threonine protein kinase, PIM1, in melanoma. Biochem Biophys Res Commun 2016;474:315-20. [Crossref] [PubMed]
- Xi RC, Biao WS, Gang ZZ. Significant elevation of survivin and livin expression in human colorectal cancer: inverse correlation between expression and overall survival. Onkologie 2011;34:428-32. [Crossref] [PubMed]
- Melucci E, Cosimelli M, Carpanese L, et al. Decrease of survivin, p53 and Bcl-2 expression in chemorefractory colorectal liver metastases may be predictive of radiosensivity radiosensivity after radioembolization with yttrium-90 resin microspheres. J Exp Clin Cancer Res 2013;32:13. [Crossref] [PubMed]
- Chu X, Chen L, Wang J, et al. SiRNA-mediated survivin inhibition enhances chemo- or radiosensivity of colorectal cancer cells in tumor-bearing nude mice. Hepatogastroenterology 2010;57:1445-52. [PubMed]
- Tuncel H, Shimamoto F, Kaneko Guangying Qi H, et al. Nuclear Aurora B and cytoplasmic Survivin expression is involved in lymph node metastasis of colorectal cancer. Oncol Lett 2012;3:1109-1114. [PubMed]
- Yoon S, Choi YC, Lee S, et al. Induction of growth arrest by miR-542-3p that targets survivin. FEBS Lett 2010;584:4048-52. [Crossref] [PubMed]
- Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008;18:997-1006. [Crossref] [PubMed]
- Wang WT, Zhao YN, Yan JX, et al. Differentially expressed microRNAs in the serum of cervical squamous cell carcinoma patients before and after surgery. J Hematol Oncol 2014;7:6. [Crossref] [PubMed]


