
Versikine, a proteolysis product of Versican: novel therapeutics for multiple myeloma
Multiple myeloma (MM) is a devastating malignancy of B cells characterized by the proliferation and accumulation of abnormal plasma cells in the bone marrow compartment (1) arising from an asymptomatic premalignant monoclonal plasma cells that are derived from post-germinal center B cells. Multistep genetic and microenvironmental changes lead to the transformation of these cells into a malignant neoplasm (2). It accounts for about 1% of neoplastic maladies and 10% of all hematologic neoplasms. Treatment for MM is focused on therapies that decrease the clonal plasma cell population and consequently decrease the signs and symptoms of disease. The melphalan-prednisolone combination therapy, immunomodulatory drugs like thalidomide and lenalidomide along with proteasome inhibitors (bortezomib) are being employed currently for the treatment of MM (3). These immunomodulatory drugs induce their effect by activation of natural killer (NK) cells, stimulation of CD4+ and CD8+ T cells and inhibition of regulatory T (T-reg) cells (4).
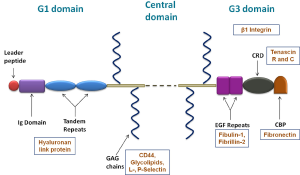
Besides the existence of these treatment regimens, various cases of MM eventually relapse due to the development of drug resistance phenomenon involving the bone marrow microenvironment. The bone marrow niche comprises of various proteoglycans with chondroitin sulphate proteoglycans forming the majority in the extracellular matrix. One of the chondroitin sulphate proteoglycan, Versican (VCAN) has gained consideration in the past few years in the context of malignancy. VCAN is a large extracellular matrix proteoglycan which provides a loose and hydrated matrix during key events in development & disease. It participates in cell adhesion, proliferation, migration, and angiogenesis, and hence, plays a role in tissue morphogenesis and maintenance (5). It consists of three domains—N-terminal domain, C-terminal domain, and the central domain where it binds to glycosaminoglycans. These domains aid in the interaction of VCAN with various factors as shown in Figure 1 (6). VCAN also found to play a key role in both malignant transformation and tumor progression. Increased VCAN expression has been observed in a wide range of malignant tumors, and has been associated with both cancer relapse and poor patient outcomes in breast, prostate, and other cancer types (7). Along with solid tumors, few reports are available in hematological malignancies such as MM, Hodgkin lymphoma and acute monocytic leukemia (8-11). Our group had previously reported the upregulation of VCAN in the bone marrow and blood of MM patients and had shown it as a potent diagnostic marker for MM (8). Apart from the role of VCAN as a marker for MM, its involvement in the progression of MM has been studied by Hope et al. in which they described the regulation of inflammatory milieu in the myeloma niche by engagement of VCAN with TLR-2/6 on the surface of myeloma–associated macrophages. This association of VCAN with TLR downstream activates tumor progression locus 2 (Tpl2) kinase contributing to myeloma progression (9).
In a recent report by Hope et al., the tolerogenic potential of VCAN has been shown to get exploited by its proteolysis product generated by digestion with ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) (12). ADAMTS1, a protease derived from mesenchymal stromal cells cleaves VCAN at Glu441-Ala442, generating a small molecule called versikine which acted as an endogenous damage-associated molecular pattern (DAMP) as studied by Hope et al. They provide evidence for this fact by exposing recombinant versikine to primary myeloma-associated macrophages and found upregulation in the levels of inflammatory cytokines (IL-6 and IL-1β) in the presence of versikine. Earlier they described that the signaling of VCAN in macrophages mediates through MAP3K (Tpl2) kinase. Hence, to study the downstream cascade of versikine, they found that loss of Tpl2 abrogated the production of IL-1β but not IL-6, confirming that IL-6 production is independent of Tpl2 kinase.
Further, they observed the induction of IL-12p40 in response to versikine resulting in M1-like phenotype (IL-12hi, IL-10lo) but concomitant addition of versikine and immune complexes switch macrophage phenotype to immunoregulatory M2B-type (IL-12lo, Il-10hi). The versikine transduced macrophages when co-cultured with human myeloma MM1.S cells leads to upregulation of type-I IFN signature genes, IFN-stimulated genes (ISGs) in both macrophages and myeloma cells. They also found induction in the expression of IFN-regulatory factors (IRFs) mainly IRF8 in myeloma cells in the presence of versikine producing macrophages making these cells prone to apoptosis.
As per the published literature, IRF8 expression gets induced via IL-27 dependent mechanism (13). In concordance with this, Hope et al. observed the upregulation of EBI3, an IL-27 subunit in both myeloma cells and primary myeloma associated macrophages in the presence of recombinant versikine. The authors also studied the co-localisation of versikine and cytotoxic cells in bone marrow biopsy of MM patients using immunohistochemistry in which they revealed that sections with high versikine expression as demonstrated by neoepitope DPEAAE441 generated by cleavage with ADAMTS1 also had high frequency of cytotoxic CD8+ T cells. Thus, the crosstalk between stromal cells and myeloid cells in the bone marrow microenvironment culminates in the production of versikine which results in immune sensing of myeloma tumors and modulating the tolerogenic potential of intact VCAN accumulation. Taken together, it could be stated that proteolysis product of VCAN, i.e., versikine, could behave as novel anti-myeloma DAMP which possess the ability to stimulate innate immunity and connecting it to adaptive immunity. This DAMP could be explored in future to generate immune response against myeloma cells and hence, may perhaps be employed therapeutically in order to potentiate T-cell–activating immunotherapies.
The study of Hope et al. paved a way to the future researchers to extend the therapeutic role of versikine against MM. The probable effect of versikine could be studied in conjunction with the currently used chemotherapeutic regimen to identify the synergistic effect observed, if any, which might be of clinical relevance and beneficial for the treatment of MM. The in vitro and ex vivo work performed by them could further be extended in myeloma xenograft model in vivo to identify the apparent involvement of the proteolysis product of VCAN in the biological system. Moreover, they described the inflammatory role of initial N-terminal product of proteolysis generated by cleavage with ADAMTS1 but C-terminal fragment obtained might also have some significant clinical involvement which could be analysed for its functionality in association with the malignancy in future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Huimin Liu (Department of lymphoma and myeloma, Institute of Hematology and Blood Disease Hospital, Chinese Academy of Medical Sciences (CAMS), Peking Union Medical College (PUMC) in Tianjin, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.67). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dimopoulos MA, Terpos E. Multiple myeloma. Ann Oncol 2010;21:vii143-50. [Crossref] [PubMed]
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011;364:1046-60. [Crossref] [PubMed]
- Chou T. Multiple myeloma: recent progress in diagnosis and treatment. J Clin Exp Hematop 2012;52:149-59. [Crossref] [PubMed]
- Mimura N, Hideshima T, Anderson KC. Novel therapeutic strategies for multiple myeloma. Exp Hematol 2015;43:732-41. [Crossref] [PubMed]
- Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol 2002;14:617-23. [Crossref] [PubMed]
- Wu YJ, La Pierre DP, Wu J, et al. The interaction of versican with its binding partners. Cell Res 2005;15:483-94. [Crossref] [PubMed]
- Du WW, Yang W, Yee AJ. Roles of versican in cancer biology--tumorigenesis, progression and metastasis. Histol Histopathol 2013;28:701-13. [PubMed]
- Gupta N, Khan R, Kumar R, et al. Versican and its associated molecules: potential diagnostic markers for multiple myeloma. Clin Chim Acta 2015;442:119-24. [Crossref] [PubMed]
- Hope C, Ollar SJ, Heninger E, et al. TPL2 kinase regulates the inflammatory milieu of the myeloma niche. Blood 2014;123:3305-15. [Crossref] [PubMed]
- Makatsori E, Lamari FN, Theocharis AD, et al. Large matrix proteoglycans, versican and perlecan, are expressed and secreted by human leukemic monocytes. Anticancer Res 2003;23:3303-9. [PubMed]
- Kischel P, Waltregny D, Greffe Y, et al. Identification of stromal proteins overexpressed in nodular sclerosis Hodgkin lymphoma. Proteome Sci 2011;9:63. [Crossref] [PubMed]
- Hope C, Foulcer S, Jagodinsky J, et al. Immunoregulatory roles of versican proteolysis in the myeloma microenvironment. Blood 2016;128:680-5. [Crossref] [PubMed]
- Mattei F, Schiavoni G, Sestili P, et al. IRF-8 controls melanoma progression by regulating the cross talk between cancer and immune cells within the tumor microenvironment. Neoplasia 2012;14:1223-35. [Crossref] [PubMed]


