
Empiric H. pylori therapy—10-day concomitant, bismuth quadruple or 14-day triple therapy: none is best
Introduction
Liou and colleagues recently reported the results of a very large randomized comparative trial of three different H. pylori therapies: 10-day concomitant, 10-day bismuth quadruple therapy, and 14-day triple therapy (1). The technical aspects of the study were truly state-of-the-art in relation to the design, execution, and data collection. Essentially all that one would like to know about the population and the variables that might have influenced outcome are presented clearly. However, planning and completing a clinical trial takes many years and investigators always risk providing answers to the questions asked may no longer be considered important. During the time the study was in progress the concepts of H. pylori treatment made a transition from a being investigated as other gastrointestinal diseases (e.g., constipation) to becoming recognized as an infectious disease (2-4). All infectious disease therapies are whenever possible susceptibility-based. Ineffective therapies are essentially never compared to state of the art therapies to prove superiority rather comparisons are designed as non-inferiority studies where all regimens provide excellent cure rates (5-7).
The authors show that, in their study, 10-day bismuth quadruple therapy was superior to 14-day triple therapy but not to 10-day concomitant therapy. The differences largely resulted from the fact that clarithromycin resistance undermines the effectiveness of clarithromycin containing therapy. However, none of the regimens was optimized in terms of doses or durations (2,8). Their conclusions are thus both population-specific and only applicable to populations with similar patterns of resistance and CYP2C19 genotype, and dose and duration specific.
They designed their study based on knowledge that triple therapy was relatively ineffective in their population and it might be proven inferior to one or both of the other therapies (9,10). While the sample size calculation proved to be reasonably accurate in predicting the outcome, it is unclear whether they divulged to the potential study candidates that, using their best available data, one of the regimens was highly likely to be inferior and that a sizable proportion of one study arm was expected to fail.
Discussion
In susceptible infections all the regimens studied when given at optimum doses and durations would be expected to achieve 95% or greater cure rates in adherent patients. Thus, fundamentally they were equal (2,3). As shown by their sample size estimation, using first principles, the outcomes could largely be predicted prior to starting the study. For example, worldwide the cure rate with 14-day clarithromycin triple therapy in the absence of clarithromycin resistance is 95% or greater. The cure rate in the presence of clarithromycin resistance depends on the effectiveness of the remaining antibiotic antisecretory combination (i.e., amoxicillin PPI dual therapy). In Asia a therapy using 30 mg of lansoprazole and 1,000 mg of amoxicillin twice a day for 14 days would be expected to cure about 40%. Clarithromycin resistance was present in 14% and thus the predicted and actual result were almost identical (slightly less than 90% cure per protocol) (11). Concomitant therapy is essentially identical to giving metronidazole and clarithromycin triple therapies simultaneously. The outcome is thus dependent on the prevalence of dual clarithromycin-metronidazole resistance which would be estimated as 4.9% based on the prevalence of clarithromycin resistance times prevalence of metronidazole resistance and if resistance to each antimicrobial was acquired independently. Dual resistance was measured as between 6% and 8%. Although data on the expected cure rate for 10-day concomitant therapy for susceptible and resistant infection are limited, one would expect a regimen that consisted of two triple therapies would cure between 90% and 95% of those with susceptible infection; increasing the duration to 14 days should further increase the cure rate by 3% or 5%. Thus, these results were also predicable.
The effectiveness of bismuth quadruple therapy is reduced by metronidazole resistance which can be partially overcome by increasing the duration of therapy and the dose of metronidazole (12,13). The investigators used full dose bismuth quadruple therapy with 2 grams of metronidazole. The duration was shorter than the recommended 14-day duration for use with metronidazole resistance (12,13). There are considerable data regarding the outcome of 7- and 14-day bismuth quadruple therapy especially at high doses. This study provided agar dilution susceptibility data which provide the most accurate estimate of metronidazole resistance and achieved excellent cure rates with resistant infection. The main issue with bismuth quadruple therapy has been with adherence. Importantly, they experienced only slightly reduced adherence which is a tribute to their willingness to spend time with the patients regarding the importance of adherence. They achieved a 96% cure rate per protocol such that increasing the duration probably might have only produced a further benefit if the proportion with metronidazole resistance had been greater (see below).
Their study opened the door for future questions that might include asking whether bismuth quadruple therapy might be further improved. There are a number studies from China and several from Italy that suggest that it might be possible to reduce the doses of bismuth and/or tetracycline in bismuth quadruple therapy to both reduce side effects and increase adherence (14-17). Examples include reducing the bismuth and tetracycline to twice a day or changing the metronidazole or tetracycline to a different third drug such as amoxicillin. Another issue is whether there is an added benefit of increasing the dose of PPI (e.g., using a double dose). This is especially interesting since the effectiveness of PPIs is greater in Asia than in the west due to a higher proportion with slow PPI metabolism and a smaller parietal cell mass. However, they presented the effects of slow vs. rapid PPI metabolizers and showed no consistent differences between the three different regimens.
Summary
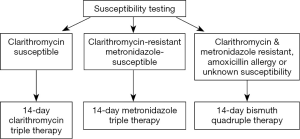
Considering that the results of this technically excellent study are population-specific and therefore of limited transferability (e.g., to another Asian population with an almost identical resistance pattern), one must ask whether it was worth giving 540 individuals clarithromycin triple therapy to achieve predictably inferior results. Overall, we conclude that there was little new information resulting from this study. We suspect that this large group of subjects were in effect likely wasted as far as advancing knowledge. How might this study be designed if being planned today? A susceptibility-based experiment might have used that data to choose therapy for those with culture results. Bismuth quadruple therapy would be given to those with dual clarithromycin metronidazole resistance, those with negative cultures, and with those with penicillin allergy (Figure 1). Such a result would have provided a road map for care that was population-independent and avoided the potential ethical issues surrounding using an established inferior regimen. It has also only recently become recognized that concomitant therapy results in all subjects receiving an unneeded antibiotic (either metronidazole or clarithromycin) and thus violates the principle of not giving an antibiotic that can provide no benefit to the patient (18). This would be avoided by susceptibility-based therapy as in illustrated in Figure 1.
Acknowledgments
Funding: Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH. Professor Yamaoka is supported in part by grants from the National Institutes of Health (DK62813) and the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (25293104, 26640114 and 15H02657).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Fengbo Tan, MD (Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, Changsha, China).
Conflicts of Interest: Dr. Graham is a paid consultant and has received research funding from RedHill Biopharma regarding novel H. pylori therapies and is a consultant to BioGaia regarding use of probiotics for H. pylori infections. Professor Yamaoka has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liou JM, Fang YJ, Chen CC, et al. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 2016;388:2355-65. [Crossref] [PubMed]
- Graham DY, Dore MP. Helicobacter pylori therapy: a paradigm shift. Expert Rev Anti Infect Ther 2016;14:577-85. [Crossref] [PubMed]
- Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology 2015;148:719-31.e3. [Crossref] [PubMed]
- Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353-67. [Crossref] [PubMed]
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital chlamydia trachomatis infection. N Engl J Med 2015;373:2512-21. [Crossref] [PubMed]
- Ghanem KG, Erbelding EJ, Cheng WW, et al. Doxycycline compared with benzathine penicillin for the treatment of early syphilis. Clin Infect Dis 2006;42:e45-9. [Crossref] [PubMed]
- Riedner G, Rusizoka M, Todd J, et al. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N Engl J Med 2005;353:1236-44. [Crossref] [PubMed]
- Malfertheiner P, Megraud F, O'Morain CA, et al. Management of helicobacter pylori infection-the maastricht v/florence consensus report. Gut 2017;66:6-30. [Crossref] [PubMed]
- Graham DY. Helicobacter pylori eradication therapy research: Ethical issues and description of results. Clin Gastroenterol Hepatol 2010;8:1032-6. [Crossref] [PubMed]
- Graham DY, Fischbach LA. Letter: the ethics of using inferior regimens in H. pylori randomised trials. Aliment Pharmacol Ther 2012;35:852-4; discussion 858. [Crossref] [PubMed]
- Graham DY. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: effect of resistance, duration, and CYP2C19 genotype. Helicobacter 2016;21:85-90. [Crossref] [PubMed]
- Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016;65:870-8. [Crossref] [PubMed]
- Graham DY, Lee SY. How to effectively use bismuth quadruple therapy: the good, the bad, and the ugly. Gastroenterol Clin North Am 2015;44:537-63. [Crossref] [PubMed]
- Lu H, Zhang W, Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: lessons from China. Eur J Gastroenterol Hepatol 2013;25:1134-40. [PubMed]
- Zhang W, Chen Q, Liang X, et al. Bismuth, lansoprazole, amoxicillin and metronidazole or clarithromycin as first-line Helicobacter pylori therapy. Gut 2015;64:1715-20. [Crossref] [PubMed]
- Dore MP, Farina V, Cuccu M, et al. Twice-a-day bismuth-containing quadruple therapy for Helicobacter pylori eradication: a randomized trial of 10 and 14 days. Helicobacter 2011;16:295-300. [Crossref] [PubMed]
- Dore MP, Maragkoudakis E, Pironti A, et al. Twice-a-day quadruple therapy for eradication of Helicobacter pylori in the elderly. Helicobacter 2006;11:52-5. [Crossref] [PubMed]
- Graham DY, Lu H, Dore MP. Empiric 4-drug non-bismuth Helicobacter pylori therapies promote misuse (overuse) of antibiotics. Helicobacter 2016;21:s139.


