
Evolving concepts of micropapillary variant urothelial carcinoma
Introduction
Micropapillary variant (MPV) urothelial carcinoma has been reported as comprising up to eight percent of contemporary urothelial carcinoma cohorts (1-4). The majority of studies have reported that MPV urothelial carcinoma portends a worse oncologic prognosis and that the tumor demonstrates more aggressive histology (3,5-8). The optimal algorithm for patients diagnosed with MPV urothelial carcinoma remains poorly defined with many researchers arguing that even in the setting of non-muscle invasive disease, these patients should be taken for early extirpative management. As MPV urothelial carcinoma remains an uncommon entity, large and multi-institutional studies have not been conducted to evaluate the efficacy of neoadjuvant or adjuvant chemotherapy. However, retrospective institutional studies have suggested that MPV demonstrates a poorer response to standard neoadjuvant chemotherapy regimens when compared with pure urothelial carcinoma (9,10). The mechanism behind this has been poorly understood. The recent article by Guo et al. begins to explore the genetic differences in MPV compared with urothelial carcinoma (11).
Immunohistochemical evaluation
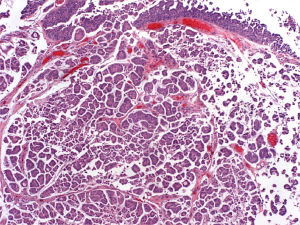
Previous immunohistochemical evaluations of MPV urothelial carcinoma have been performed to determine the best markers to identify MPV in bladder cancer specimens. Figure 1 demonstrates an H & E stain of MPV urothelial carcinoma. GATA 3 (GATA binding protein 3) and uroplakin 3 have been reported as reliable markers for urothelial tumors although the sensitivity of uroplakin 3 is worse than for GATA 3. GATA 3 is a member of a family of transcription factors involved in embryogenesis and has been reported to be the most sensitive and specific for bladder cancer (12,13). Recent studies have reported that GATA 3 levels in MPV urothelial carcinoma are similar to levels in pure urothelial carcinoma (14). Interestingly, while GATA 3 levels are similar between MPV and pure urothelial carcinoma, GATA 3 levels have been reported to be significantly lower in other variants of urothelial carcinoma such as squamous differentiation variant and sarcomatoid variant (14,15). The reason for this difference is unclear but is likely more reflective of changes in the squamous differentiation variant.
An additional marker of urothelial carcinoma is p63. Wang et al. recently reported that the presence of p63 was an independent predictor of worse survival in patients with urothelial carcinoma who underwent radical cystectomy with urinary diversion (16). Despite the majority of patients with pure urothelial carcinoma displaying expression of p63, recent studies have reported decreased p63 expression in patients with MPV, with between 27% and 54% of MPV tumors staining positive for p63 (15,17).
Choi et al. previously reported that muscle-invasive urothelial carcinoma can be divided into luminal, p53-like luminal, and basal subtypes which were predictive of response to chemotherapy and overall tumor behavior (18). The pure urothelial carcinoma cases with the basal subtype had overexpression of p63 and were more aggressive at presentation. These patients were additionally more sensitive to traditional neoadjuvant chemotherapy regimens (18,19). The luminal subtype demonstrated increased PPAR-γ expression and FGFR mutations. The p53-like luminal subtype tumors shared PPAR-γ expression and FGFR mutations but were notably chemo-resistant to current neoadjuvant chemotherapy regimens (18).
In the recent study by Guo et al., it was reported that whereas in the pure urothelial carcinoma cohort 47.2% were basal subtype, 24.7% were luminal subtype, and 28.1% were p53-like luminal subtype, when examining the MPV urothelial carcinoma cohort, 2.3% were basal subtype (n=1), 51.2% were luminal subtype (n=22), and 46.5% were p53-like luminal subtype (n=20) (11). The MPV urothelial carcinoma tumors demonstrated, almost uniformly, GATA 3 and uroplakin 2. Furthermore, the tumors demonstrated increased PPAR-γ expression and downregulation of p63. When examining the response to chemotherapy among MPV tumors, 66% (n=4/6) of tumors in the luminal subtype and 45% (n=5/11) of tumors in the p53-like luminal subtype group demonstrated response to neoadjuvant chemotherapy, similar to prior studies suggesting that the p53-like luminal subtype was less likely to respond to chemotherapy.
Genetic alterations
Downregulation of miR-296, which is associated with upregulation of over 300 downstream genes, was found to be a driver in the expression of MPV in the recent study by Guo et al. (11). This may be a critical pathway that could be targeted to better identify patients with this uncommon variant of urothelial carcinoma. Downregulation of miR-296 has previously been reported to be associated with aggressive changes in other cancers including prostate cancer (20-23). As part of miR-296 downregulation, the RUVBL1 pathway is activated. This is known to be associated with genes that play critical roles in metastasis, cell growth, and DNA repair. Additionally, RUVBL1 acts via p53 to block p53 mediated cellular apoptosis (24). Furthermore, as the RUVBL1 pathway has been noted to be associated with poor response to traditional chemotherapy, it may serve as the mechanism of resistance to cisplatin based regimens. Both miR-296 and the RUVBL1 pathway could be intervened upon to prevent the aggressive changes seen with MPV urothelial carcinoma.
An additional potential intervenable pathway identified by the Guo et al. study is PPAR-γ (11). The study found that the majority of MPV urothelial carcinoma tumors, regardless of p53-like subset, demonstrate upstream PPAR-γ expression. PPAR-γ has been postulated as a target for muscle invasive bladder cancer and research is ongoing into its clinical relevance as a therapeutic target (25,26). Troglitazone, a PPAR-γ agonist, induces apoptosis and autophagy in bladder cancer cells (27); although more research is needed before these agents are used in clinical practice.
Clinical implications
A particularly interesting finding in the Guo et al. study is the fact that when examining tumors with MPV sections and pure urothelial carcinoma sections, the molecular signatures of the urothelial carcinoma sections were similar to the MPV sections (11). This finding would imply that regardless of the percentage of MPV in a tumor specimen, the patient will likely have a more aggressive clinical progression of disease. Previously, authors have suggested that in the setting of only small volume variant histology (<5%), a patient could potentially be treated as if their tumor were pure urothelial carcinoma; however, the current study would suggest that these patients may be more similar to the higher volume MPV patients than previously understood and may benefit from early radical cystectomy with urinary diversion until new chemotherapeutic or immunomodulating agents are identified.
The lack of responsiveness to current chemotherapy regimens and molecular alterations indicative of an aggressive tumor suggest that patients with MPV urothelial carcinoma may benefit from early extirpative management. The approach to patients with MPV urothelial carcinoma will continue to evolve as new molecular targets are identified. As previously discussed, miR-296, RUVBL1, and PPAR-γ are potential targets that could revolutionize the way MPV urothelial carcinoma is approached.
Conclusions
MPV urothelial carcinoma remains an uncommon variant of bladder cancer that can be challenging to treat. Studies such as that by Guo et al. are landmark in building an understanding of the fundamental changes that occur in the development of MPV urothelial carcinoma. With subsequent studies of the molecular underpinnings and evaluation of therapeutic targets, management of patients with MPV will be revolutionized.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Peng Zhang (Department of Urology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.12.56). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cheng L, Lopez-Beltran A, Bostwick DG. Bladder Pathology. Hoboken, NJ, USA: Wiley-Blackwell, 2012.
- Lopez-Beltran A, Montironi R, Blanca A, et al. Invasive micropapillary urothelial carcinoma of the bladder. Hum Pathol 2010;41:1159-64. [Crossref] [PubMed]
- Monn MF, Kaimakliotis HZ, Pedrosa JA, et al. Contemporary bladder cancer: variant histology may be a significant driver of disease. Urol Oncol 2015;33:18.e15-20. [Crossref] [PubMed]
- Watts KE, Hansel DE. Emerging concepts in micropapillary urothelial carcinoma. Adv Anat Pathol 2010;17:182-6. [Crossref] [PubMed]
- Pokuri VK, Syed JR, Yang Z, et al. Predictors of Complete Pathologic Response (pT0) to Neoadjuvant Chemotherapy in Muscle-invasive Bladder Carcinoma. Clin Genitourin Cancer 2016;14:e59-65. [Crossref] [PubMed]
- Kamat AM, Dinney CP, Gee JR, et al. Micropapillary bladder cancer: a review of the University of Texas M. D. Anderson Cancer Center experience with 100 consecutive patients. Cancer 2007;110:62-7. [Crossref] [PubMed]
- Ghoneim IA, Miocinovic R, Stephenson AJ, et al. Neoadjuvant systemic therapy or early cystectomy? Single-center analysis of outcomes after therapy for patients with clinically localized micropapillary urothelial carcinoma of the bladder. Urology 2011;77:867-70. [Crossref] [PubMed]
- Masson-Lecomte A, Xylinas E, Bouquot M, et al. Oncological outcomes of advanced muscle-invasive bladder cancer with a micropapillary variant after radical cystectomy and adjuvant platinum-based chemotherapy. World J Urol 2015;33:1087-93. [Crossref] [PubMed]
- Willis DL, Porten SP, Kamat AM. Should histologic variants alter definitive treatment of bladder cancer? Curr Opin Urol 2013;23:435-43. [Crossref] [PubMed]
- Willis DL, Fernandez MI, Dickstein RJ, et al. Clinical outcomes of cT1 micropapillary bladder cancer. J Urol 2015;193:1129-34. [Crossref] [PubMed]
- Guo CC, Dadhania V, Zhang L, et al. Gene Expression Profile of the Clinically Aggressive Micropapillary Variant of Bladder Cancer. Eur Urol 2016;70:611-620. [Crossref] [PubMed]
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol 2012;36:1472-6. [Crossref] [PubMed]
- Ellis CL, Chang AG, Cimino-Mathews A, et al. GATA-3 immunohistochemistry in the differential diagnosis of adenocarcinoma of the urinary bladder. Am J Surg Pathol 2013;37:1756-60. [Crossref] [PubMed]
- Liang Y, Heitzman J, Kamat AM, et al. Differential expression of GATA-3 in urothelial carcinoma variants. Hum Pathol 2014;45:1466-72. [Crossref] [PubMed]
- Paner GP, Annaiah C, Gulmann C, et al. Immunohistochemical evaluation of novel and traditional markers associated with urothelial differentiation in a spectrum of variants of urothelial carcinoma of the urinary bladder. Hum Pathol 2014;45:1473-82. [Crossref] [PubMed]
- Wang L, Zhou M, Feng C, et al. Prognostic value of Ki67 and p63 expressions in bladder cancer patients who underwent radical cystectomy. Int Urol Nephrol 2016;48:495-501. [Crossref] [PubMed]
- Lotan TL, Ye H, Melamed J, et al. Immunohistochemical panel to identify the primary site of invasive micropapillary carcinoma. Am J Surg Pathol 2009;33:1037-41. [Crossref] [PubMed]
- Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014;25:152-65. [Crossref] [PubMed]
- Kiselyov A, Bunimovich-Mendrazitsky S, Startsev V. Key signaling pathways in the muscle-invasive bladder carcinoma: Clinical markers for disease modeling and optimized treatment. Int J Cancer 2016;138:2562-9. [Crossref] [PubMed]
- Lee KH, Lin FC, Hsu TI, et al. MicroRNA-296-5p (miR-296-5p) functions as a tumor suppressor in prostate cancer by directly targeting Pin1. Biochim Biophys Acta 2014;1843:2055-66.
- Zaravinos A, Radojicic J, Lambrou GI, et al. Expression of miRNAs involved in angiogenesis, tumor cell proliferation, tumor suppressor inhibition, epithelial-mesenchymal transition and activation of metastasis in bladder cancer. J Urol 2012;188:615-23. [Crossref] [PubMed]
- Vaira V, Faversani A, Dohi T, et al. miR-296 regulation of a cell polarity-cell plasticity module controls tumor progression. Oncogene 2012;31:27-38. [Crossref] [PubMed]
- Wei JJ, Wu X, Peng Y, et al. Regulation of HMGA1 expression by microRNA-296 affects prostate cancer growth and invasion. Clin Cancer Res 2011;17:1297-305. [Crossref] [PubMed]
- Taniue K, Oda T, Hayashi T, et al. A member of the ETS family, EHF, and the ATPase RUVBL1 inhibit p53-mediated apoptosis. EMBO Rep 2011;12:682-9. [Crossref] [PubMed]
- Conconi D, Sala E, Bovo G, et al. Using Copy Number Alterations to Identify New Therapeutic Targets for Bladder Carcinoma. Int J Mol Sci 2016;17:271. [Crossref] [PubMed]
- Mansure JJ, Nassim R, Chevalier S, et al. A novel mechanism of PPAR gamma induction via EGFR signalling constitutes rational for combination therapy in bladder cancer. PLoS One 2013;8:e55997 [Crossref] [PubMed]
- Yan S, Yang X, Chen T, et al. The PPARγ agonist Troglitazone induces autophagy, apoptosis and necroptosis in bladder cancer cells. Cancer Gene Ther 2014;21:188-93. [Crossref] [PubMed]


