
Targeting the pyrimidine synthesis pathway for differentiation therapy of acute myelogenous leukemia
One of the key features of acute leukemia is the differentiation arrest at early progenitor stage of hematopoietic maturation. This has led to the search for therapeutic agents that can overcome this differentiation block to promote terminal differentiation. The first and most notable clinical success to date has been treatment with all-trans retinoic acid (ATRA) for acute promyelocytic leukemia (APL), which accounts for 10% of acute myeloid leukemias (AML) (1).
Given the genetic complexity of AML, it has been difficult to extend this approach to other leukemia subtypes. Recently however, Sykes et al. (2) reported a powerful approach to identifying differentiating agents that show promise for leukemia therapy. This study focused on leukemia driven by the homeobox transcription factor Hoxa9, which is overexpressed in the majority of AML cases and represents a commonly dysregulated pathway among leukemias with various driver mutations, such as MLL-fusion, NUP98-translocation and NPM1c mutations (3). To model this differentiation arrest, cell lines were generated using an inducible form of Hoxa9, where an estrogen receptor is fused with Hoxa9 (ER-Hoxa9) so that the fusion protein is only active in the presence of beta-estradiol (E2). The activated fusion protein allows murine bone marrow cells to proliferate indefinitely as granulocytes-macrophage progenitor (GMP) cells in vitro. Upon withdrawal of E2, the cells undergo synchronized terminal differentiation into neutrophils. To facilitate high throughput screening for differentiation, the authors also took advantage of a transgenic mouse model with the GFP gene knocked into the lysozyme locus (lysozyme-GFP), which is only expressed in mature myeloid cells.
A library of 330,000 small molecules was tested for their ability to induce differentiation of the lysozyme-GFP-ER-Hoxa9 myeloblasts (Figure 1). Of this library, 12 compounds demonstrated reproducible differentiation in concentration-response assays. Upon testing with additional cell lines, C03 and C07, two structurally unrelated compounds, were selected for further study based on their potency in inducing differentiation that phenocopied Hoxa9 inactivation in both murine and human AML models.
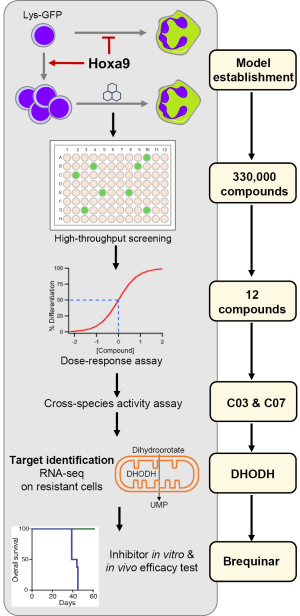
To identify the protein targets of the two compounds, Sykes et al. employed a genetic target-identification approach: the cell lines were cultured in the presence of slowly increasing concentrations of C03 or C07 over an extended period of time until they acquired resistance. Cross-resistance to C03 and to C07 was found, suggesting similar resistance mechanisms. The authors then performed expression profiling as well as whole-exome sequencing, in order to analyze the genomic and transcriptomic alterations in the resistant cell lines. Strikingly, only eight genes were commonly up regulated, all of which are located within a 100 kb region on murine chromosome 8. One of the eight genes encoded dihydroorotate dehydrogenase (DHODH), a conserved enzyme involved in de novo biosynthesis of pyrimidines across different species. Importantly, C03 and C07 were found to be inhibitors of DHODH in an in vitro enzyme inhibition assay.
Next, Sykes et al. explored the importance of pyrimidine synthesis inhibition on overcoming the differentiation block in several ways. First, since DHODH is involved in the intracellular synthesis of uridine, extracellular uridine was added to the cultures and was found to abrogate the effect of both C03 and C07 treatments in a dose-dependent manner. Second, treatment with pyrazofurin, a potent small-molecule inhibits an enzyme downstream of DHODH in the same uridine synthesis pathway, was also found to induce myeloid differentiation of cells immortalized by Hoxa9. Perhaps most remarkably, all but 1 of the 12 hits in this small-molecule screening was confirmed as DHODH inhibitors, highlighting the dependence on the pyrimidine biosynthesis pathway of AML cells.
Finally, the authors tested activity of DHODH inhibition in vivo leukemia models, using a highly bioavailable DHODH-specific inhibitor, brequinar sodium (BRQ). In various Hoxa9-driven AML models, including murine and human cell lines and patient-derived xenografts, BRQ induced cellular differentiation, reduced leukemia burden, and prolonged disease-free survival for primary and secondary transplantation. Importantly, BRQ was well tolerated by the mice at doses that were effective for slowing AML progression.
In the era of precision medicine, the “one-fit-all” therapies with which AMLs have been treated for over 30-year await revolutionary improvement. This discovery by Sykes et al. is exciting and hopeful, particularly because it offers promise for a new therapeutic approach for the majority of AML that is associated with HOXA9 overexpression. It is noteworthy that DHODH has recently been independently identified as important for proliferation of AML cell lines using a CRISPR “dropout” screen (4), corroborating the validity of targeting this enzyme for AML treatment. The precise cellular target for DHODH remains to be identified, and this will be a logical next step in refining this therapy. In addition, since the cells developed resistance after prolonged DHODH inhibition, further studies will be needed to identify pathways of chemotherapy resistance and what combinations of other agents can overcome this resistance.
In addition, this study illustrates the power of focusing on a genetically well-defined common pathway in cancer, in this case HOXA9 overexpression and using a reporter for high throughput detection of differentiation inducing agents. The results demonstrate that experimentally induced resistance coupled with expression profiling can be a powerful way of deconvoluting data from phenotypic screens to identify new therapeutic pathways. These strategies can be employed to facilitate future studies in the mechanisms and interventions of leukemia and other malignancies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xiaoying Zhou (Institute of Gastroenterology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.28). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nowak D, Stewart D, Koeffler HP. Differentiation therapy of leukemia: 3 decades of development. Blood 2009;113:3655-65. [Crossref] [PubMed]
- Sykes DB, Kfoury YS, Mercier FE, et al. Inhibition of Dihydroorotate Dehydrogenase Overcomes Differentiation Blockade in Acute Myeloid Leukemia. Cell 2016;167:171-186.e15. [Crossref] [PubMed]
- Collins CT, Hess JL. Role of HOXA9 in leukemia: dysregulation, cofactors and essential targets. Oncogene 2016;35:1090-8. [Crossref] [PubMed]
- Tzelepis K, Koike-Yusa H, De Braekeleer E, et al. A CRISPR Dropout Screen Identifies Genetic Vulnerabilities and Therapeutic Targets in Acute Myeloid Leukemia. Cell Rep 2016;17:1193-205. [Crossref] [PubMed]


