
Neuroendocrine differentiation in prostate cancer: key epigenetic players
A recent publication by Dardenne et al. (1) in Cancer Cell (October 10, Vol. 30: 563–577, 2016) demonstrate N-Myc overexpression and its stabilization by Aurora-A (AURKA) and AKT1 Kinase induces enhancer of zeste homolog 2 (EZH2)-mediated transcriptional reprogramming repressing androgen receptor (AR) and driving neuroendocrine prostate cancer (1). This has important clinical implication proposing combinatorial therapy using inhibitors of phosphatidylinositol 3-kinase (PI3K)-AKT and AURKA as future trials in the management of neuroendocrine tumors.
Neuroendocrine differentiation in prostate cancer (NEPC) is frequently associated with advance disease and poor clinical outcome (2,3). The incidence of neuroendocrine phenotypes in primary prostate cancers is approximately 1% whereas in lethal metastatic castrate-resistant prostate cancers its percentage is up to 25–30% (4). Emerging evidence suggests that NEPC develops as a transdifferentiation from prostate adenocarcinoma, in response to androgen deprivation therapy and/or treatment with inhibitors targeting AR signaling pathways (5). During NEPC development, cells lose their granular structure and demonstrate small cell neuroendocrine-like morphology, positive for typical neuroendocrine markers such as chromogranins, synaptophysin (SYP), and neurospecific enolase (NSE) but little or very low levels of AR and AR-regulated gene expression (6). Since AR signaling is required for epithelial cell differentiation during prostate development, inhibition of AR pathway likely initiates developmental reprogramming of prostate adenocarcinoma to neuroendocrine tumors through a transdifferentiation mechanism (7). Unlike prostate adenocarcinoma, neuroendocrine tumors are very aggressive with median cancer-specific survival is less than 2 years (8). Conventionally, neuroendocrine prostate cancers have been managed clinically with cisplatin-based chemotherapy regimens, however further identification and understanding of the oncogenic drivers of this phenotype necessitates the development of novel targeted therapies.
Dardenne et al. (1) performed an integrated analysis employing clinical prostate cancer specimens consisting of NEPC tumor cells and castrate-resistant prostate tumors with focal neuroendocrine differentiation, which correlated higher N-Myc expression in the neuroendocrine phenotype. In parallel studies, authors generated genetically-engineered mouse (GEM) model that harbor a CAG-driven lox-stop-lox human MYCN gene integrated into the ROSA26 (LSL-MYCN) locus and a Tmprss2-driven tamoxifen-activated Cre recombinase. T2-Cre specifically mediates Cre recombination in luminal cells in the prostate. These mice were cross-bred with other mice that harbor a Pten conditional knockout allele leading to increase in PI3K/AKT signaling enhancing N-Myc protein stability. Mice with heterozygous loss of Pten and N-Myc overexpression exhibited a divergent mixture of large invasive carcinoma in the prostate with foci containing AR-positive adenocarcinoma or AR-negative NEPC tumor cells, compared with the corresponding control. The NEPC foci displayed high levels of MYCN RNA with N-Myc overexpression associated with enhanced AKT signaling. In another study, Lee et al. (9) using a model of premalignant prostatic intraepithelial neoplasia demonstrated that active myristoylated AKT1 together with forced expression of MYCN in normal prostate basal cells (but not in luminal cells) led to the development of invasive, metastatic castration-resistant tumors positive for neuroendocrine markers. Furthermore, NEPC tumors with N-Myc overexpression abrogates AR signaling and downregulates AR-target gene FKBP5, a member of the immunophilin protein family, which serves as a scaffolding protein for AKT and PHLPP promoting PHLPP dephosphorylation of AKT at amino acid S473 (p-AKT). FKBP5 downregulation leads to activation of AKT by releasing FKBP5-PHLPP-mediated suppression of AKT in the crosstalk between AR signaling and PI3K-AKT pathway. Based on the results, the current study establishes the oncogenic role of MYCN in neuroendocrine prostate cancer. Several published studies have demonstrated the oncogenic role of various genes that likely facilitate the progression of prostate adenocarcinoma to NEPC through AR suppression (5,7), involvement of AR-splice variants (10), and induction of neuroendocrine features and neural programs (11). Many genes associated with a neuroendocrine phenotype, include AR-regulated genes viz. ARG2 (12) and hASH-1 (13). Additional drivers in the pathogenesis of NEPC include loss of tumor suppressors, activation of mitotic programs, and genomic instability. Tumor suppressors TP53 and RB1 are dysfunctional in various malignancies including NEPC (14,15). The combined deficiency of RB1 and TP53 promotes the transformation to NEPC, as reported in conditional mouse models of NEPC (16). Besides, several cell-cycle genes have been shown to be frequently amplified and/or overexpressed in NEPC, thus supporting their role in driving uncontrolled NEPC growth and proliferation. This list includes UBE2C, cyclin D1, Src family kinase-FYN, and polo-like kinase PLK1Jeny (17-19).
The most noteworthy observation of the study involves binding of N-Myc to AR enhancers and the subsequent interaction of this complex with the members of polycomb repressive complex 2 (PRC2). The polycomb group proteins EZH2, suppressor of zeste 12 (SUZ12), embryonic ectoderm development (EED), and RbAp48 form the PRC2 complex, which specifically trimethylates lysine-27 of histone H3 (H3K27) on target gene promoters (20). This histone marker is part of a preprogrammed cellular memory system inherited through mitotic cell divisions and thus preserves cellular identity. Genome-wide location analysis revealed that PRC2 represses a special set of developmental regulators and signaling molecules. Notably, EZH2 is the catalytic subunit of PRC2 and is a highly conserved histone methyltransferase (HMT) that targets H3K27 trimethylation. This trimethylated H3K27 chromatin is commonly associated with silencing of differentiation genes in humans (21). Current study reveals that the catalytic activity of EZH2 facilitates N-Myc/AR/EZH2-PRC2 complex formation. High levels of EZH2 protein and its activity in the prostate of mice overexpressing N-Myc redirects EZH2 activity to N-Myc target gene promoters resulting in transcription repression, whereas EZH2 inhibition reverses N-Myc gene regulation (1). Thus, the interaction between N-Myc, AR, and EZH2 results in the abrogation of AR signaling despite abundant levels of AR, suggesting that the amplification of MYCN and inactivation of AR might be a significant phenomenon in NEPC tumors (Figure 1). Moreover, RE1-silencing transcription factor (REST) is another epigenetic alteration essential in driving the development of the NE phenotype, which is controlled by AR (22). In addition to these epigenetic modifications, several novel somatic mutations in multiple chromatin/histone modifiers including MLL2, UTX and ASXL1 as well as transcription factors FOXA1 and ETS2 were noted in neuroendocrine prostate tumors (23).
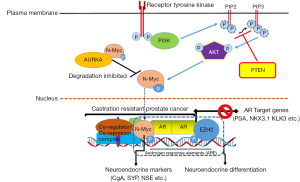
Using cell culture and pre-clinical mouse models, the authors further demonstrate that N-Myc forms a complex with AURKA that results in N-Myc stabilization in NEPC tumors. Aurora kinases (A and B), composed of serine/threonine kinase, are essential for cell proliferation. Specifically, AURKA amplification has been reported in over 60% of prostate cancer from patients that developed treatment-related NEPC and in 86% of metastases, whereas concurrent amplification of N-Myc was present in 69% of treated prostate cancer and 83% of metastases (24). In the experiments, pharmacological inhibition of allosteric AURKA inhibitor led to rapid dissociation of the N-Myc-AURKA complex and rapid degradation of N-Myc. In accordance with these findings, Lee et al. (9) evaluated the therapeutic efficacy of several AURKA inhibitors and found that CD532, a novel AURKA inhibitor, results in a significant reduction in MYCN protein levels and decreased tumor burden in pre-clinical models driven by MYCN overexpression. Interestingly, this effect was not observed with MLN8237—an AURKA inhibitor, which is currently in early-phase clinical trials, as downregulation of MYCN protein levels did not appear to be dependent on AURKA kinase activity. However, the present study findings demonstrate that N-Myc overexpressing cells exhibit higher sensitivity to AKT inhibitors in combination with allosteric AURKA inhibitor.
Clinical and genomic profiling data propose that neuroendocrine prostate cancers may originate de novo from a small population of neuroendocrine cells present in the prostate (2-4). However in the majority of cases these tumors diverge from a population of luminal-derived metastatic castrate-resistant adenocarcinoma. Either way, these two processes are preferentially mobilized under selective pressure from castration and/or treatment with AR inhibitors in conjunction with genetic perturbations that help initiate or maintain NE phenotype. While the current study establishes the oncogenic role of N-Myc in NEPC, it is still unclear the precise cell of origin of NEPC and their maintenance in vivo. It is postulated that the stimulation of growth factor production, such as epidermal growth factor, insulin-like growth factor, keratinocyte growth factor and secretion of some pro-inflammatory cytokines including interleukin: IL-6, IL-8, IL-1β and macrophage migration inhibitory factor can promote neuroendocrine differentiation through AR pathway in the absence of androgens (25).
The significance of this manuscript lies in its ability to demonstrate the cooperation between N-Myc and EZH2 in repressing AR to drive NEPC. This has important clinical implication and rationale for combining therapy targeting or co-targeting molecules in N-Myc driven tumors through newly designed clinical trials in future.
Acknowledgments
Funding: This work was supported by VA Merit Review 1I01BX002494 and the United States Public Health Service Grants RO1CA108512, R21CA193080 and R03CA186179 to S Gupta.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Lichao Sun (State Key Laboratory of Molecular Oncology, National Cancer Center (NCC)/Cancer Hospital, Chinese Academy of Medical Sciences (CAMS), Peking Union Medical College, Beijing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.20). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dardenne E, Beltran H, Benelli M, et al. N-Myc Induces an EZH2-Mediated Transcriptional Program Driving Neuroendocrine Prostate Cancer. Cancer Cell 2016;30:563-77. [Crossref] [PubMed]
- Santoni M, Conti A, Burattini L, et al. Neuroendocrine differentiation in prostate cancer: novel morphological insights and future therapeutic perspectives. Biochim Biophys Acta 2014;1846:630-7.
- Grigore AD, Ben-Jacob E, Farach-Carson MC. Prostate cancer and neuroendocrine differentiation: more neuronal, less endocrine? Front Oncol 2015;5:37. [Crossref] [PubMed]
- Epstein JI, Amin MB, Beltran H, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol 2014;38:756-67. [Crossref] [PubMed]
- Lipianskaya J, Cohen A, Chen CJ, et al. Androgen-deprivation therapy-induced aggressive prostate cancer with neuroendocrine differentiation. Asian J Androl 2014;16:541-4. [Crossref] [PubMed]
- Ather MH, Abbas F, Faruqui N, et al. Correlation of three immunohistochemically detected markers of neuroendocrine differentiation with clinical predictors of disease progression in prostate cancer. BMC Urol 2008;8:21. [Crossref] [PubMed]
- Komiya A, Yasuda K, Watanabe A, et al. The prognostic significance of loss of the androgen receptor and neuroendocrine differentiation in prostate biopsy specimens among castration-resistant prostate cancer patients. Mol Clin Oncol 2013;1:257-62. [PubMed]
- Bostwick DG, Qian J, Pacelli A, et al. Neuroendocrine expression in node positive prostate cancer: correlation with systemic progression and patient survival. J Urol 2002;168:1204-11. [Crossref] [PubMed]
- Lee JK, Phillips JW, Smith BA, et al. N-Myc Drives Neuroendocrine Prostate Cancer Initiated from Human Prostate Epithelial Cells. Cancer Cell 2016;29:536-47. [Crossref] [PubMed]
- Qiao J, Grabowska MM, Forestier-Roman IS, et al. Activation of GRP/GRP-R signaling contributes to castration-resistant prostate cancer progression. Oncotarget 2016;7:61955-69. [PubMed]
- Bishop JL, Thaper D, Vahid S, et al. The Master Neural Transcription Factor BRN2 Is an Androgen Receptor-Suppressed Driver of Neuroendocrine Differentiation in Prostate Cancer. Cancer Discov 2017;7:54-71. [Crossref] [PubMed]
- Gannon PO, Godin-Ethier J, Hassler M, et al. Androgen-regulated expression of arginase 1, arginase 2 and interleukin-8 in human prostate cancer. PLoS One 2010;5:e12107 [Crossref] [PubMed]
- Rapa I, Volante M, Migliore C, et al. Human ASH-1 promotes neuroendocrine differentiation in androgen deprivation conditions and interferes with androgen responsiveness in prostate cancer cells. Prostate 2013;73:1241-9. [Crossref] [PubMed]
- Tan HL, Sood A, Rahimi HA, et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res 2014;20:890-903. [Crossref] [PubMed]
- Hansel DE, Nakayama M, Luo J, et al. Shared TP53 gene mutation in morphologically and phenotypically distinct concurrent primary small cell neuroendocrine carcinoma and adenocarcinoma of the prostate. Prostate 2009;69:603-9. [Crossref] [PubMed]
- Akamatsu S, Wyatt AW, Lin D, et al. The Placental Gene PEG10 Promotes Progression of Neuroendocrine Prostate Cancer. Cell Rep 2015;12:922-36. [Crossref] [PubMed]
- Pernicová Z, Slabáková E, Fedr R, et al. The role of high cell density in the promotion of neuroendocrine transdifferentiation of prostate cancer cells. Mol Cancer 2014;13:113. [Crossref] [PubMed]
- Gururajan M, Cavassani KA, Sievert M, et al. SRC family kinase FYN promotes the neuroendocrine phenotype and visceral metastasis in advanced prostate cancer. Oncotarget 2015;6:44072-83. [PubMed]
- Grobholz R, Griebe M, Sauer CG, et al. Influence of neuroendocrine tumor cells on proliferation in prostatic carcinoma. Hum Pathol 2005;36:562-70. [Crossref] [PubMed]
- Clermont PL, Lin D, Crea F, et al. Polycomb-mediated silencing in neuroendocrine prostate cancer. Clin Epigenetics 2015;7:40. [Crossref] [PubMed]
- Deb G, Singh AK, Gupta S. EZH2: not EZHY (easy) to deal. Mol Cancer Res 2014;12:639-53. [Crossref] [PubMed]
- Svensson C, Ceder J, Iglesias-Gato D, et al. REST mediates androgen receptor actions on gene repression and predicts early recurrence of prostate cancer. Nucleic Acids Res 2014;42:999-1015. [Crossref] [PubMed]
- Gupta S, Li J, Kemeny G, et al. Whole Genomic Copy Number Alterations in Circulating Tumor Cells from Men with Abiraterone or Enzalutamide-Resistant Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 2011;1:487-95. [Crossref] [PubMed]
- Yuan TC, Veeramani S, Lin MF. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer 2007;14:531-47. [Crossref] [PubMed]


