
The association between expression of hypoxia inducible factor-1α and multi-drug resistance of acute myeloid leukemia
Introduction
Acute myeloid leukemia (AML) is the most common form of acute leukemia affecting adults. Approximately 10,500 new cases are recorded each year in the United States, and this condition accounts for 1.2% of all cancer deaths in the United States (1). Although hematopoietic stem cell transplantation has been widely employed in clinical settings, chemotherapy remains the first line of treatment for AML. However, failure of chemotherapy and post-remission relapse of leukemia likely occur mainly because of multi-drug resistance (MDR). The number of remaining leukemic cells is related to recurrence (2). Cancer cells may exhibit MDR induced by a specific drug, but cancer cells may become resistant to several other anti-cancer drugs with different structures and mechanisms (3). Various hypotheses have been presented, but the mechanisms of MDR remain unclear (4).
Hypoxic tumor microenvironment can affect the susceptibility to chemotherapy drugs via the following mechanisms (5). First, hypoxia can stimulate the aggressiveness of neoplasms and induce distant metastasis. Second, the cell cycle of tumors can be slowed down or even terminated by hypoxia. Hypoxia likely weakens the cytotoxicity of chemotherapeutic drugs because these drugs elicit strong cytotoxic effects on actively proliferating cells. Extracellular pH becomes lower than intracellular pH if hypoxia occurs; as such, a transmembrane pH gradient can be established. Under these circumstances, the aggregation of alkaline drugs, such as Adriamycin, in cells can be inhibited. As a result, the resistance of tumor cells to radiotherapy and chemotherapy increases and curative effects are impaired.
As a relevant transcription factor in hypoxia induction, hypoxia inducible factor-1α (HIF-1α) is involved in various hypoxia-induced target gene expression. HIF-1α is considered a new anti-neoplasm target overproduced in tumor tissues and participates in the oncogenesis, development, and progression of tumors. HIF-1α functions through several steps, including protein expression, dimerization, combination to target DNA sequences, and transcriptional activation. The whole process is regulated by many factors. For example, PI3K/Akt and MAPK signal transduction pathways are implicated in HIF-1α protein expression and HIF-1transcriptional activation, respectively (6). Numerous post-transcriptional modification enzymes, including deubiquitinase and phosphatase, stimulate protein expression and regulate HIF-1α activity. However, HIF-1α production can decrease when these signal pathways are inhibited. Various compounds, such as PD98059 signal pathway inhibitor, Wortmannin, Topotecan, and other molecular compounds, including 2ME2 and YC-1, can inhibit HIF-1α. With enhanced understanding of the biological mechanisms of cellular adaptation in hypoxic environments, novel strategies can be developed to treat neoplasms (7,8).
HIF-1α is related to the MDR of patients with different kinds of tumors. For instance, HIF-1α mediates the MDR of osteosarcoma by activating the P-glycoprotein (P-gp) expression and by regulating the expression of c-Myc and p21 (9). In the meantime, HIF-1α plays a major role in the MDR of laryngeal cancer cells with similar mechanisms (10). However, the role of HIF-1α in the MDR of malignant hematological tumors remains unclear. Few studies have been carried out to evaluate the correlation between HIF-1α and MDR of hematological malignancy. Therefore, this study aimed to investigate the correlation of HIF-1α and other hypoxia-induced gene expression with the MDR of patients with AML and to discuss the potential underlying mechanisms, which could possibly provide additional information for AML treatment.
Methods
Patient characteristics
A total of 118 patients with AML (apart from M3) in accordance with French-American-Britain (FAB) criteria but without a history of other cancers or hematological malignancies were enrolled in the study from January 2010 to November 2015. Donors signed the questionnaire and informed consent form in accordance with Chinese guidelines for blood donation. This study was also conducted in accordance with the declaration of Helsinki and was approved by the Ethics Committee of Drum Tower Hospital affiliated with Nanjing University.
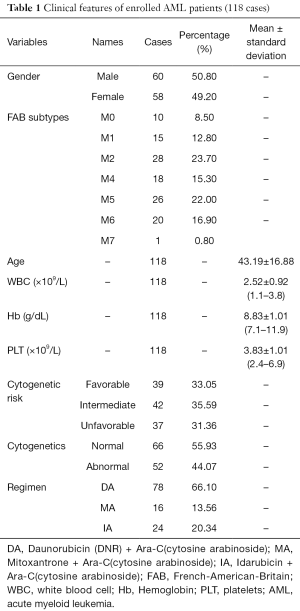
Of the total number of the enrolled patients with AML, 10 had M0 (minimally differentiated AML), 17 had M1 (AML without maturation), 28 had M2 (AML with maturation), 18 had M4 (acute myelomonocytic), 26 had M5 (acute monocytic leukemia), 20 had M6 (erythroleukemia), and 1 had M7. The characteristics of the patients are shown in Table 1. Cytogenetic risk groups were defined as follows, by WHO criteria: unfavorable, –7/del(Jeny7q), –5/del(Jeny5q), abn3q, abn9q, abn11q, abn-17p; favorable, inv(Jeny16)/t(Jeny16;Jeny16)/del(Jeny16q), and t(Jeny8;Jeny21); and intermediate risk, all other karyotypic aberrations or a normal karyotype (11).
Full table
Evaluation of chemotherapy regimens and therapeutic response
All of the patients with AML accepted cytosine arabinoside-based chemotherapy, which included Mitoxantrone + Ara-C(cytosine arabinoside) (MA), Daunorubicin (DNR) +Ara-C(cytosine arabinoside) (DA), and Idarubicin + Ara-C (IA) regimens (Table 2). Of the 118 patients, 78 were treated with DA induction chemotherapy, 24 were subjected to IA induction chemotherapy, and 16 patients were administered with MA induction chemotherapy regimen. Complete response (CR) was defined as the absolute values of granular leukocytes and platelets in peripheral blood higher than 1.5×109/L and 100×109/L, respectively; blast cell count in the bone marrow was less than 5% for at least 4 weeks. The symptoms and signs of leukemia disappeared. Partial remission (PR) required similar criteria, with the exception of the presence of 6–20% marrow blasts. Non-remission (NR) was described on the basis of three clinical manifestations, including blood analysis, bone marrow biopsy inconsistent with the standard criteria for CR, and >20% promyelocytic cells in the bone marrow. Early death was noted if a patient died within 8 weeks from the start of the first induction of the therapeutic course. CR and PR were considered good responses, and NR and early death were recorded as poor response (12).

Full table
RNA extraction and quantitative real-time polymerase chain reaction (PCR)
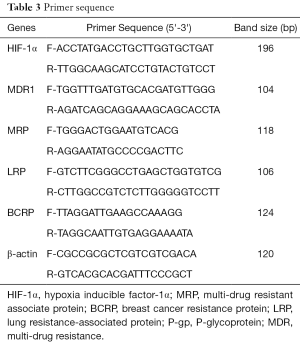
RNA was isolated from the blood samples of the participants and stored at −20 °C for later use. The RNA was then reverse-transcribed to obtain cDNA. PCR primers were designed by Primer Premier 6.0 (Table 3). The experiments were performed in triplicate and normalized to β-actin. PCR was performed under the following conditions: 40 cycles with a three-cycle loop in each cycle; initial temperature at 95 °C for 2 min; followed by 95 °C for 10 s, 60 °C for 3 s, and 70 °C for 45 s. The program lasted 1 min at 60 °C.
Full table
Statistics
Data were statistically analyzed in SPSS v12.0 (SPSS Inc., Chicago, IL, USA). The correlation of the HIF-1α expression with MDR1, multi-drug resistant associate protein (MRP), breast cancer resistance protein (BCRP), and lung resistance-associated protein (LRP) gene expression was analyzed through Pearson’s χ2. Significant differences of the HIF-1a expression among various responders were calculated with least-significant difference analysis. Survival curves were plotted with Kaplan-Meier method. In all of the tests, P value of <0.05 was considered statistically significant.
Results
Clinical features of the enrolled patients with AML
The clinical features of the enrolled patients are presented in Table 1. The patients’ ages ranged from 15 to 81 years, and the median patient age was 43.19±16.88 (14–78) years. The Patients with AML included 60 males and 58 females, and the male-to-female ratio was close to 1:1 (50.80% and 49.20%). Most of the enrolled patients had M2 (23.70%), and a few of the patients had M0 (8.5%). The mean of white blood cell (WBC), Hb, and PLT counts were 2.52±0.92×109/L, 8.83±1.01 g/dL, and 3.83±1.01×109/L, respectively. In terms of cytogenetic risk, 39 patients exhibited favorable cytogenetic risk, 42 patients manifested intermediate risk, and 37 patients showed unfavorable risk. The effective rates of the chemotherapeutic methods used in this study did not significantly vary (P>0.05). Hence, the efficacy of different chemotherapy regimens did not significantly differ.
Analysis of the mRNA expression Of HIF-1a, MDR1, BCRP, MRP, and LRP in the patients with AML and the correlation between expression and chemotherapy
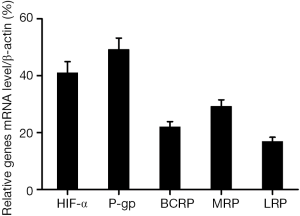
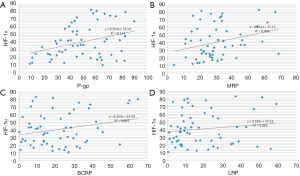
RT-qPCR revealed that the median mRNA expression levels of HIF-1α, MDR1, BCRP, MRP, and LRP were 40.81%±21.60%, 48.74%±20.61%, 21.67%±15.76%, 30.31%±14.10%, and 17.99%±15.12%, respectively (Figure 1). The correlation coefficients of HIF1-α, MDR1, BCRP, MRP, and LRP were 0.354, 0.333, 0.451, and 0.182, respectively. These results indicated that HIF-1α could be significantly correlated with (Table 4; Figure 2A,B; P<0.05) the expression of MDR1 (r2=0.114) and MRP (r2=0.086). By contrast, HIF-1α was not significantly associated (Table 5; Figure 2C,D; P>0.05) with the expression of BCRP (r2=0.059) and LRP (r2=0.016). Therefore, HIF-1α was correlated with the mRNA expression of drug-resistant genes, namely, MDR1 and MRP, in patients with AML after chemotherapy.

Full table

Full table
Effects of the mRNA expression of HIF-1a, MDR1, BCRP, MRP, and LRP on the survival rates of patients
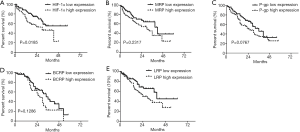
The evaluation of the mRNA expression of HIF-1α in different chemotherapy regimens indicated that DA regimen (41.32±20.44) was more efficient than HA regimen (49.49±22.27) and MA regimen (25.31±20.32; Table 5; P<0.05). Kaplan-Meier test was performed to estimate the survival rate of the patients on the basis of HIF-1α expression. The patients with low HIF-1α expression showed a significantly higher survival rate than those with high HIF-1α expression (HR =4.63; Figure 3A; P<0.05). Nevertheless, the expression levels of MDR1 (HR =0.35), BCRP (HR =0.406), MRP (HR =0.413), and LRP (HR =0.49) were not associated with the survival rate of patients with AML (Figure 3B-E; P>0.05). Therefore, high HIF-1α expression at a transcription level is related to the prognosis of patients with AML after chemotherapy.
Discussion
Hypoxia is considered the main cause of MDR and chemotherapy failure; however, the underlying mechanisms are complicated. (I) The bone marrow of patients with AML is in a state of chronic physical hypoxia, recent study demonstrated sever hypoxia of 1% O2 could induce decreased cellular proliferation which cause S phase significantly decreased, this mechanism is thought to be related with cytarabine resistant because the cytotoxic activity is linked to the proliferative activity and hence the numbers of cells in the S phase (13); (II) the structural and functional abnormality of vessels in solid tumors and tumor necrosis can induce insufficient blood flow, which prevents drugs to reach and kill tumor cells; (III) hypoxia can promote the proliferation of tumor cells through rapid cell division and thus reduce tumor chemo-sensitivity; (IV) the intracellular and extracellular distribution of anti-cancer drugs can be affected by changes in pH of tumor cells, while pH of tissues under low oxygen conditions is lower than pH of normal tissue (14); (V) hypoxia increases the expression of MDR gene, P-gp, MRP, BCRP, and LRP (15). These mechanisms illustrate that hypoxia is associated with MDR of tumor cells.
HIF-1α is overexpressed in pancreatic cancer cells, especially under hypoxic conditions; the inhibition of HIF-1α can induce a more efficient response to chemotherapy; therefore, HIF-1α expression is related to the therapeutic effect of pancreatic cancer (16). For example, a recombinant lentivirus containing HIF-1α small interfering RNA (siRNA) can reverse the MDR of breast cancer xenograft models by downregulating the expression of HIF-1α; this finding suggested that HIF-1α also participates in MDR of breast cancer (17). It is also be found that patients with advanced colon carcinoma and with expressed HIF-1α and P-gp are more resistant to chemotherapy than those without HIF-1α and P-gp expressed in their system. The sensitivity of chemotherapy can be restored after the HIF-1α gene in colon cancer cells is silenced. The inhibition of HIF-1α can significantly decrease the mRNA or protein expression of MDR1/P-gp in colon cancer cells; therefore, MDR1/P-gp expression is associated with the expression of HIF-1α (18).
Although the correlation between HIF-1α and MDR in solid tumors has been comprehensively described, the correlation between these factors in hematological malignancy remains unclear. Nevertheless, our study demonstrated that the HIF-1α expression was correlated with MDR in AML. Therefore, HIF-1α may be implicated in MDR of hematological malignancy. However, further studies should be conducted to clarify the underlying mechanisms of this phenomenon.
Our results demonstrated that HIF-1 can be used as an independent indicator of the prognosis of tumor patients because this indicator can transmit cellular hypoxia signals and induce “hypoxic effect.” As an important hypoxia-induced transcription factor, HIF-1 participates in angiogenesis, anaerobic metabolism, chemotherapy and radiotherapy resistance, and tumor cell invasion and metastasis (19). Increased HIF-1 expression can be considered as an indicator of poor prognosis of different solid tumors (20-22). Recent study also suggested that the HIF-1 expression is related to the poor survival of patients with AML (23). In our study, Kaplan-Meier curve analysis suggested that the post-chemotherapy survival rate of patients with AML was significantly related to HIF-1α expression (P<0.05), and the DSS of patients with low HIF-1α expression was longer than those with high HIF-1α expression. This finding indicated that HIF-1α could be considered as a prognosis indicator of patients with AML. Our study also indicated that the MDR-associated proteins, including MDR1, BCRP, MRP, and LRP, were not significantly correlated with the survival rate of patients with AML. This finding showed that MDR may not directly cause poor prognosis, and HIF-1α expression might be accounted for the poor prognosis of AML through other mechanisms. Recent researches demonstrated that high HIF-1α expression is associated with the extramedullary infiltration of AML, and this process can be considered as a possible mechanism (24). Nevertheless, the mechanisms of the relationship between HIF-1α expression and poor prognosis of AML should be further investigated.
Acknowledgments
We would like to thank all the volunteers who took part in this study.
Funding: This work was supported by the National Natural Science Foundation of China (81400162, 81570174), the Natural Science Foundation of Jiangsu Province (BK20140100), and the Technique Development Foundation of Nan Jing (Outstanding Youth Foundation, JQX15004).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.10). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Drum Tower Hospital affiliated with Nanjing University (No. 2010-11) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Thomas A, Murray T, et al. Cancer statistics, 2002. CA Cancer J Clin 2002;52:23-47. [Crossref] [PubMed]
- Brisco MJ, Sykes PJ, Dolman G, et al. Early resistance to therapy during induction in childhood acute lymphoblastic leukemia. Cancer Res 2000;60:5092-6. [PubMed]
- Pérez-Tomás R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr Med Chem 2006;13:1859-76. [Crossref] [PubMed]
- Vaupel P, Thews O, Hoeckel M. Treatment resistance of solid tumors: role of hypoxia and anemia. Med Oncol 2001;18:243-59. [Crossref] [PubMed]
- Mahoney BP, Raghunand N, Baggett B, et al. Tumor acidity, ion trapping and chemotherapeutics. I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol 2003;66:1207-18. [Crossref] [PubMed]
- Airley RE, Monaghan JE, Stratford IJ. Hypoxia and disease: opportunities for novel diagnostic and therapeutic prodrug strategies. The Pharmaceutical Journal 2000;264:666-73.
- Seddon BM, Honess DJ, Vojnovic B, et al. Measurement of tumor oxygenation: in vivo comparison of a luminescence fiber-optic sensor and a polarographic electrode in the p22 tumor. Radiat Res 2001;155:837-46. [Crossref] [PubMed]
- Marx J. Cell biology. How cells endure low oxygen. Science 2004;303:1454-6. [Crossref] [PubMed]
- Roncuzzi L, Pancotti F, Baldini N. Involvement of HIF-1α activation in the doxorubicin resistance of human osteosarcoma cells. Oncol Rep 2014;32:389-94. [PubMed]
- Li DW, Dong P, Wang F, et al. Hypoxia induced multidrug resistance of laryngeal cancer cells via hypoxia-inducible factor-1α. Asian Pac J Cancer Prev 2013;14:4853-8. [Crossref] [PubMed]
- Barragan E, Collado M, Cervera J, et al. The GST deletions and NQO1*2 polymorphism confers interindividual variability of response to treatment in patients with acute myeloid leukemia. Leuk Res 2007;31:947-53. [Crossref] [PubMed]
- Diamond HR, Ornellas MH, Orfao A, et al. Acute myeloid leukemia of donor origin after allogeneic stem cell transplantation from a sibling who harbors germline XPD and XRCC3 homozygous polymorphisms. J Hematol Oncol 2011;4:39. [Crossref] [PubMed]
- Drolle H, Wagner M, Vasold J, et al. Hypoxia regulates proliferation of acute myeloid leukemia and sensitivity against chemotherapy. Leuk Res 2015;39:779-85. [Crossref] [PubMed]
- Helmlinger G, Yuan F, Dellian M, et al. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med 1997;3:177-82. [Crossref] [PubMed]
- Comerford KM, Wallace TJ, Karhausen J, et al. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 2002;62:3387-94. [PubMed]
- He X, Wang J, Wei W, et al. Hypoxia regulates ABCG2 activity through the activivation of ERK1/2/HIF-1α and contributes to chemoresistance in pancreatic cancer cells. Cancer Biol Ther 2016;17:188-98. [Crossref] [PubMed]
- Li S, Wei Q, Li Q, et al. Down-regulating HIF-1α by lentivirus-mediated shRNA for therapy of triple negative breast cancer. Cancer Biol Ther 2015;16:866-75. [Crossref] [PubMed]
- Chen J, Ding Z, Peng Y, et al. HIF-1α inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PLoS One 2014;9:e98882 [Crossref] [PubMed]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003;3:721-32. [Crossref] [PubMed]
- Wu XH, Qian C, Yuan K. Correlations of hypoxia-inducible factor-1α/hypoxia-inducible factor-2α expression with angiogenesis factors expression and prognosis in non-small cell lung cancer. Chin Med J (Engl) 2011;124:11-8. [PubMed]
- Noguera R, Fredlund E, Piqueras M, et al. HIF-1alpha and HIF-2alpha are differentially regulated in vivo in neuroblastoma: high HIF-1alpha correlates negatively to advanced clinical stage and tumor vascularization. Clin Cancer Res 2009;15:7130-6. [Crossref] [PubMed]
- Matsuyama T, Nakanishi K, Hayashi T, et al. Expression of hypoxia-inducible factor-1alpha in esophageal squamous cell carcinoma. Cancer Sci 2005;96:176-82. [Crossref] [PubMed]
- Deeb G, Vaughan MM, McInnis I, et al. Hypoxia-inducible factor-1α protein expression is associated with poor survival in normal karyotype adult acute myeloid leukemia. Leuk Res 2011;35:579-84. [Crossref] [PubMed]
- Chen P, Jiang X, Huang HF, et al. Expression of HIF-1α in primary acute myeloid leukemia cells and its relationship with prognosis. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2015;23:19-23. [PubMed]


