
Zoledronic acid as potential efficacy application combined with icotinib for non-small cell lung cancer with bone metastases
Introduction
Lung cancer is the leading causes of cancer death throughout the world. Morbidity and mortality are both increasing in both developing and developed countries (1,2). Non-small-cell lung cancer (NSCLC) remains the most common kind of lung cancer which accounts for 80–90% (3). For patients with metastasis NSCLC, the prevalence of bone metastases in the course of disease ranges between 30% and 40%, and approximately 65% of cases are found at diagnosis (4,5). The bone metastases in NSCLC is related to a reduced survival and median overall survival (mOS) about 6–12 months (6).
Bisphosphonate (BP) therapy is usually used in patients with bone metastases to prevent complications (7). Zoledronic acid (ZOL) is a third generation bisphosphonate. Recent studies suggested that ZOL might have a function to prevent proliferation of cancer cells and inducing apoptosis to some extent (8). And some studies demonstrated that ZOL might provide clinically antitumor advantage in patients with bone metastases (9-11). We also carried out a retrospective study to show survival benefit of ZOL treatment in bone metastases of NSCLC patients (12).
Patients with epidermal growth factor receptor (EGFR) mutations have good response to EGFR tyrosine kinase inhibitors (TKIs) (13). Icotinib is a kind of EGFR-TKI which has been successfully employed as therapy for EGFR activating mutations, such as erlotinib and gefitinib (14). Two studies reported that ZOL improves the inhibitory effects of gefitinib and delay the progression of disease on EGFR-mutated cancer cells (15,16). So, combining treatment of EGFR-TKI with ZOL may develop a more effective for NSCLC with bone metastasis patients and inhibit TKI resistance. However, few studies showed clinically survival benefits according to this therapy in NSCLC patients with bone metastasis.
In our study, we proposed to evaluate the efficacy of zoledronic acid (ZOL) combining with icotinib on patients with bone metastases of NSCLC, particularly in EGFR mutation.
Methods
Study population
A total of 171 patients were diagnosed by pathologic histology of advanced NSCLC from July 2011 to May 2015 at Zhejiang Cancer Hospital, were administrated with icotinib treatment. The inclusive criteria were confirmed bone metastases by emission computed tomography (ECT), magnetic resonance imaging (MRI) or computed tomography (CT) at initial presentation, and receive ZOL treatment at least once. Patients who received icotinib had shown clinical benefits (e.g., CR, PR, SD) and treatment ≥6 months. We collected data on blood routine, biochemical and electrolyte were examined at regular intervals. This study was approved by the Institutional Review Board of Zhejiang Cancer Hospital and the ID/number of ethics approval was IRB-(2016) 135.
Treatment and evaluation of efficacy
Patients received 4 mg of ZOL as a period of 15-min infusion every 3–4 weeks. Icotinib treatment was administered as 125 mg oral in tablet from three times per day. The tumor efficacy was assessed by the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, including complete remission (CR), partial remission (PR), stable disease (SD), and progression disease (PD). The patients were evaluated the time of effect or progression, by CT of the chest and abdomen, MRI and other staging procedures. Patients were assessed the therapeutic effect after 28 days with icotinib and every two months to evaluate the disease.
Follow-up and statistical analysis
Patient follow-up by outpatient or telephone was done until January 19, 2016. We retrospectively studied 210 patients. And after follow-up, 184 patients were followed up. And survival time of 13 patients was shorter than 12 months. So the remaining 171 patients were analyzed. Progression free survival (PFS) was defined as from initiation of icotinib treatment to treatment failure or death from any cause. Overall survival (OS) time was calculated from the day of diagnosis NSCLC with bone metastases to the date of death or the last follow-up. The impact of the potential variables affecting PFS and OS was assessed by univariate analysis with the log-rank tests. Multivariate testing was done by the Cox regression analyses. Statistical analysis was performed using SPSS software, version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics of patients
The baseline characteristics are showed in Table 1. The median age was 61 years. There were 91 males and 80 females. 36.8% (63/171) had a smoking history. A total of 34 (19.9%) patients were with brain metastasis. A total of 133 (77.8%) had EGFR mutations (72 with exon 19 deletion, 59 with L858R mutation in exon 21 and 2 with exon 18 G719X mutation). 38 (22.2%) patients had not do EGFR mutation test. 49.1% (84/171) patients had received icotinib as first line treatment. Of the 171 patients, 127 (74.3%) received ZOL more than 6 months, 93 (54.4%) received ZOL more than 1 years and 25 (14.6%) received more than 2 years. Among 25 patients who received ZOL were more than 2 years, 9 patients didn’t continue ZOL treatment, and 16 patients still continued using a period of every 21-28 days. Then, 34 patients (19.9%) were with ZOL treatment between 6 months and 1 year, the remaining 44 patients (25.7%) were shorter than 6 months. In 16 patients, no one had happened sever adverse events including osteonecrosis of the jaw (ONJ), grade 3 or 4 hypocalcaemia, or an increase in serum creatinine levels.
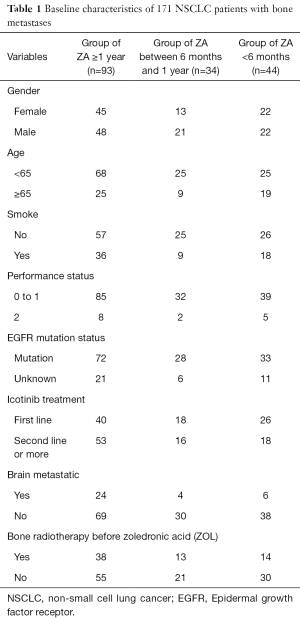
Full table
Clinical efficacy and survival analysis
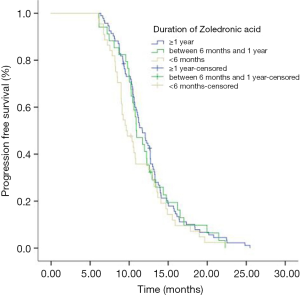
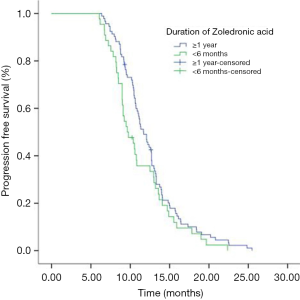
Eighty-seven (50.9%) patients were PR and 49.1% (84/184) patients were SD in the initial icotinib therapy. Median PFS of all patients during icotinib treatment was 11.0 months. The PFS of icotinib as first line treatment was 12.5 and 10.6 months as second line or more, respectively (P<0.001). Median OS time for all patients was 24.6 months. The mPFS in group of ≥1 year, group of between 1 year and 6 months, and group of <6 months ZOL treatment were 11.7, 10.9 and 9.6 months respectively (P=0.224) (Figure 1). The mPFS in ≥1 year and <6 months ZOL group were 11.7 and 9.63 months (P=0.094) (Figure 2). Median OS in three groups were 24.6, 24.4 and 23.0 months, respectively (P=0.498). And the mOS in the group of ≥1 years ZOL was longer than the group of <6 months ZOL treatment (24.6 vs. 23.0 months, P=0.217). There were 46 patients were survival longer than 2 years and 25 patients received ZOL treatment ≥2 years. The mPFS in the group of ≥2 years ZOL and the group of 1 year ≤ ZOL treatment <2 years were 12.7 vs. 12.4 months, P=0.930). The mOS were 26.1 months and 24.8 months (P=0.864).
The incidence of bone pain in group of ZOL treatment between ≥1 year (A) and <1 year (B)
The cumulative incidences of bone pain before ZOL treatment were 49.4% and 46.4% in group of ≥1 year (A) and <1 year (B). When after 6 months and 12 months, the incidence of bone pain at Group A was 6.9% and 20.7%, respectively. 7.1% and 28.6% in Group B (Table 2).

Full table
Skeletal Related Event (SREs) in group of ZOL treatment between ≥1 year (A) and <1 year (B)
Bone radiation therapy occurred most often among SREs, followed by surgical stabilization, spinal cord compression or pathologic fracture. According to the incidence of SREs, 36 of the 87 patients in Group A (41.4%) and 24 of the 84 patients in Group B (28.6%) experienced SREs before ZOL treatment (P=0.079). In group A, 34 patients received bone radiotherapy, 1 patient happened spinal cord compression and 1 patient happened pathologic fracture. In group B, 22 patients received bone radiotherapy, 1 patient received surgical stabilization and 1 patient happened pathologic fracture. During ZOL treatment, the incidence rate of SREs in group A and B were 17.2% (15/87) and 14.3% (12/84), respectively (P=0.596) (Table 3). In group A, 15 patients received bone radiotherapy, 1 patient received surgical stabilization. In group B, 10 patients received bone radiotherapy, 2 patients received surgical stabilization.

Full table
Zoledronic acid (ZOL) treatment and survival in 133 EGFR mutated patients
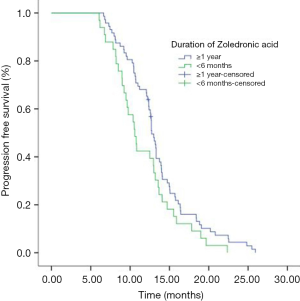
Median PFS of 133 patients during icotinib treatment was 12.5 months. The mPFS of icotinib as first line therapy was 13.2 months and 11.0 months as second line or more, respectively (P=0.001). Median OS of 133 patients was 25.8 months. The mPFS in group of ≥1 year, group of between 1 year and 6 months, and group of <6 months ZOL treatment were 12.7, 11.0 and 10.6 months respectively (P=0.203). The mPFS in ≥1 year and <6 months ZOL group were 12.7 months and 10.6 months (P=0.055) (Figure 3). Median OS in three groups were 26.1 months, 25.5 months and 25.6 months, respectively (P=0.669). The multivariate analyses showed that brain metastasis (P=0.001), icotinib treatment line (P<0.001) and duration of ZOL (P=0.002) were found to significantly influence PFS, and only brain metastasis (P=0.026) was found to significantly influence OS (Table 4).

Full table
Discussion
In recent years, EGFR-TKIs have become an essential treatment for advanced or metastatic EGFR mutated NSCLC patients. Some studies reported that the efficacy of EGFR-TKIs to treatment NSCLC with bone metastases. Satoh et al. (17) showed a case of lung adenocarcinoma with bone metastasis that a complete response was achieved after gefitinib treatment. For patients with bone metastases can cause worse survival and may reduce the quality of life. Some reports showed that ZOL has its direct effects on tumor cells, its antitumor effect by activating γδ T cells and by sensitizing tumors to γδT cell-mediated cytotoxicity to proliferate and secrete interferon-γ (18). Feng et al. (15) reported that the effect of gefitinib combining with ZOL on EGFR mutated tumor cells of NSCLC. ZOL improved the anticancer activities of gefitinib on these cells and delayed TKI resistance in vitro. However, there are no clinical reports about the synergism of EGFR-TKIs with ZOL. In addition, there have been no studies involving combined icotinib with ZOL in clinical studies. In our report, we present our analysis with ZOL therapy in combination with EGFR-TKI for NSCLC patients with bone metastases.
Zarogoulidis et al. (10) showed a study of 144 patients that ZOL prolonged overall survival (OS) in patients with bone metastases of NSCLC (578 vs. 314 days; P<0.001). Kosaka et al. (19) reported a case of lung adenocarcinoma with multiple bone metastases that showed a gradual complete response to combined administration of erlotinib and ZOL. It demonstrated that combined treatment with both drugs is effective against bone metastases. We ever retrospectively analyzed ZOL treatment in advanced non-small cell lung cancer patients with bone metastases. 109 patients received ZOL more than 6 times, and the other 204 patients received ZOL <6 times. Survival time was significantly longer in ≥6 times (385 vs. 275 days; P=0.002) (12). In this present report, we defined much longer duration of ZOL treatment. The results showed median PFS in ≥1 year ZOL group was slightly longer than <6 months ZOL treatment (11.7 vs. 9.6 months P=0.094), although not statistically significant. In addition, 133 EGFR mutated patients, mPFS in ≥1 year was longer than <6 months ZOL group (12.7 vs. 10.6 months, P=0.055). And the multivariate analyses revealed that the duration of ZOL therapy an independent predictor to PFS. Hence, we thought that long time ZOL treatment (≥1 year) might provide a potential positive effect and a moderate prolong survival time for NSCLC patients with bone metastases. On the other hand, for survival time exceeding two year, mPFS (12.7 vs. 12.4 months, P=0.930) and mOS (26.1 and 24.8 months, P=0.864) were neither significant difference between 1 year ZOL treatment and 2 years. Therefore, after 1 year ZOL treatment, using period of ZOL could be adjusted according to the situation of patients and extension of cycle. In the future, it needs prospective and random clinical researches to decide appropriate duration of ZOL therapy and when to stop using ZOL therapy.
Bone pain is usually the first symptom of lung cancer with bone metastases and the incidence is about 80% (20). It would become worse with disease progression, and influence the quality of patients’ life (5). In an Italy study, it revealed that almost 50% of participants happened bone pain and affected their daily activities (21). In our report, before ZOL treatment, the cumulative incidences of bone pain were 49.4% and 46.4% in group of ≥1 year and <1 year respectively. The incidences of bone pain at 6 months were 6.9% and 7.1%, respectively. At 12 months, the occurrence rates were 20.7% in ≥1 year group and 28.6% in <1 year group. The pain had not been worse during ZOL therapy and might increase the effect to relieve pain.
Skeletal-related events (SREs) are defined as bone radiotherapy due to bone metastases, pathologic fracture, surgery to bone and spinal cord compression (22). In a 21-month study, the frequency of SREs in lung cancer with bone metastases patients who did not receive bone-targeted agents ranged from 40% to 52% (23). Another study in a large US population reported that SREs were present in 22% at diagnosis of bone metastasis, with a cumulative incidence of 59% in lung cancer patients (24). These events typically occur around periods of disease progression, becoming more frequent as the disease becomes more extensive (5). In our report, 41.4% patients in ≥1 year treatment and 28.6% patients in <1 year treatment experienced SREs before ZOL treatment. During ZOL treatment, the incidence of SREs in ≥1 year and <1 year treatment were 17.2% and 14.3%, respectively. This outcome showed that ZOL might play an important role in delaying or reducing the risk of SREs. In recent years, the median survival of patients with advanced disease has increased more than 1 year, particularly in EGFR mutated patients. The metastases to bone would become more common, and the frequency of SREs may increase. Therefore, there is a need to consider treatments that can reduce the risk of SREs.
In addition, our research had several limitations. First, the nature of retrospective study will induce the statistical bias. And a small number of patients (23.9%) had not been detected EGFR status, it might influence statistical differences. To date, there are no clinical studies to focus on duration of ZOL treatment with EGFR-TKI drug. Our retrospective analysis would be considered a certain clinical meaningful.
Conclusions
We have demonstrated the combination of ZOL and icotinib therapy may have modest activity application for non-small cell lung cancer with bone metastases patients. ZOL might also reduce the risk of skeletal-related events during the treatment course.
Acknowledgments
Funding: This work was supported by grants from the Natural Science Foundation of Zhejiang (No. LY13H160024) and fund of Chinese medical science and technology ministry of health (2015ZB020).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.36). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Zhejiang Cancer Hospital and the ID/number of ethics approval was IRB-(2016) 135. Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Houston KA, Henley SJ, Li J, et al. Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004-2009. Lung Cancer 2014;86:22-8. [Crossref] [PubMed]
- Tsuya A, Kurata T, Tamura K, et al. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer 2007;57:229-32. [Crossref] [PubMed]
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s-9s. [Crossref] [PubMed]
- Kuchuk M, Addison CL, Clemons M, et al. Incidence and consequences of bone metastases in lung cancer patients. J Bone Oncol 2013;2:22-9. [Crossref] [PubMed]
- De Marinis F, Eberhardt W, Harper PG, et al. Bisphosphonate use in patients with lung cancer and bone metastases: recommendations of a European expert panel. J Thorac Oncol 2009;4:1280-8. [Crossref] [PubMed]
- Neville-Webbe HL, Gnant M, Coleman RE. Potential anticancer properties of bisphosphonates. Semin Oncol 2010;37:S53-65. [Crossref] [PubMed]
- Lipton A, Cook R, Saad F, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer 2008;113:193-201. [Crossref] [PubMed]
- Zarogoulidis K, Boutsikou E, Zarogoulidis P, et al. The impact of zoledronic acid therapy in survival of lung cancer patients with bone metastasis. Int J Cancer 2009;125:1705-9. [Crossref] [PubMed]
- Costa L, Cook RJ, Body J, et al. Zoledronic Acid Treatment Delays Disease Progression and Improves Survival in Patients With Bone Metastases From Solid Tumors and Elevated Levels of Bone Resorption. Available online: https://www.researchgate.net/publication/295815477_Zoledronic_Acid_Treatment_Delays_Disease_Progression_and_Improves_Survival_in_Patients_With_Bone_Metastases_From_Solid_Tumors_and_Elevated_Levels_of_Bone_Resorption
- Song Z, Zhang Y. Zoledronic acid treatment in advanced non-small cell lung cancer patients with bone metastases. Med Oncol 2014;31:898. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. Non-Small Cell Lung Cancer, Version 6.2015. J Natl Compr Canc Netw 2015;13:515-24. [PubMed]
- Hu X, Han B, Gu A, et al. A single-arm, multicenter, safety-monitoring, phase IV study of icotinib in treating advanced non-small cell lung cancer (NSCLC). Lung Cancer 2014;86:207-12. [Crossref] [PubMed]
- Feng C, Liu X, Li X, et al. Zoledronic acid increases the antitumor effect of gefitinib treatment for non-small cell lung cancer with EGFR mutations. Oncol Rep 2016;35:3460-70. [PubMed]
- Chang JW, Hsieh JJ, Shen YC, et al. Bisphosphonate zoledronic acid enhances the inhibitory effects of gefitinib on EGFR-mutated non-small cell lung carcinoma cells. Cancer Lett 2009;278:17-26. [Crossref] [PubMed]
- Satoh H, Ishikawa H, Ohara G, et al. Prolonged response to gefitinib in bone metastasis. Med Oncol 2009;26:101-2. [Crossref] [PubMed]
- Noguchi A, Kaneko T, Kamigaki T, et al. Zoledronate-activated Vγ9γδ T cell-based immunotherapy is feasible and restores the impairment of γδ T cells in patients with solid tumors. Cytotherapy 2011;13:92-7. [Crossref] [PubMed]
- Kosaka T, Yamaki E, Mogi A, et al. A case of lung adenocarcinoma with postoperative recurrence of multiple bone metastases that showed a gradual complete response to combined administration of erlotinib and zoledronic acid. Tumori 2014;100:e45-8. [PubMed]
- Kosteva J, Langer C. The changing landscape of the medical management of skeletal metastases in nonsmall cell lung cancer. Curr Opin Oncol 2008;20:155-61. [Crossref] [PubMed]
- Di Maio M, Gridelli C, Gallo C, et al. Prevalence and management of pain in Italian patients with advanced non-small-cell lung cancer. Br J Cancer 2004;90:2288-96. [PubMed]
- Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 2004;96:879-82. [Crossref] [PubMed]
- Hirsh V, Tchekmedyian NS, Rosen LS, et al. Clinical benefit of zoledronic acid in patients with lung cancer and other solid tumors: analysis based on history of skeletal complications. Clin Lung Cancer 2004;6:170-4. [Crossref] [PubMed]
- Oster G, Lamerato L, Glass AG, et al. Natural history of skeletal-related events in patients with breast, lung, or prostate cancer and metastases to bone: a 15-year study in two large US health systems. Support Care Cancer 2013;21:3279-86. [Crossref] [PubMed]


