
Progress in gastrointestinal cancer immunotherapy
Introduction
Immune checkpoint inhibitors (ICIs) have demonstrated dramatic efficacy in a range of malignancies, gaining approval initially in melanoma and non-small cell lung cancer (NSCLC), and more recently in bladder and head and neck cancers (1). These novel antibodies block inhibitory T cell membrane receptor “checkpoints” like cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death protein 1 (PD-1), enhancing anti-tumor immunity (2). The first FDA approved agent in this class, ipilimumab (Yervoy®, Bristol-Myers Squibb Company, New York City, USA), was shown in two randomized controlled trials to improve overall survival (OS) and objective response rate (ORR) when compared to the gp100 peptide vaccine or dacarbazine chemotherapy, respectively, in advanced melanoma (3,4). The PD-1 inhibitor nivolumab (Opdivo®, Bristol-Myers Squibb Company) also demonstrated improved outcomes in melanoma patients (5,6). In addition, two significant phase III trials of nivolumab showed increased OS in squamous and non-squamous NSCLC compared with docetaxel (7,8) and improved OS compared to everolimus (Afinitor®, Novartis Pharmaceutical Corp., Basel, Switzerland) in advanced renal cell carcinoma (9). On par breakthrough results were achieved with the PD-1 inhibitor pembrolizumab (Keytruda®, Merck Sharp & Dohme Corp., New Jersey, USA), which demonstrated prolonged OS and progression-free survival (PFS) when compared to ipilimumab in advanced melanoma (10) and similar noteworthy results in advanced NSCLC (11). Novel combination regimens of PD-1 and CTLA-4 inhibitors have now taken center stage as potentially more efficacious options for advanced melanoma and lung cancer (12,13), with new innovative combination strategies in a host of additional malignancies currently under investigation (14).
Against the backdrop of rapid ICI success in melanoma and NSCLC, the role of ICIs in gastrointestinal (GI) cancers has been defined more slowly. In general, ICI single agent therapy has been ineffective in GI cancers. However, sub-groups of GI cancers with microsatellite instability high (MSI-high) have experienced impressive responses to single agent ICIs. Furthermore, subsets of patients with gastric and/or gastro-esophageal junction (GEJ) cancer with programmed death-ligand 1 (PD-L1) positive tumors have benefited from ICI therapy. At the same time, immature data involving ICIs have accrued in other GI cancers, specifically pancreatic, biliary and anal cancer. Currently, new therapeutic approaches involving synergistic strategies of immunomodulatory agents (including DNA/peptide-based vaccines, ICIs, etc.), molecular-based targeted therapy, and anti-neoplastic therapy are under preliminary investigation in GI cancers. This review summarizes the fundamental studies defining the role of ICIs in gastric, GEJ, colorectal, pancreatic, biliary and anal cancers.
Gastric and esophageal cancer
Gastric and esophageal carcinogenesis involves mucosa-associated inflammatory changes often secondary to either Helicobacter pylori chronic gastritis (15) or reflux-induced intestinal metaplasia, respectively (16). The pro-inflammatory milieu driving the progressive evolution from metaplasia to neoplasia can concomitantly induce up-regulation of checkpoint receptor ligand PD-L1 in an estimated 40% of gastric and esophageal cancers respectively (17). A wealth of clinical data suggest that increased tumoral PD-L1 expression may be a predictive biomarker of treatment response to ICIs (18). Moreover, detailed sequencing of gastric cancers has found more than 30% to have more than 192 nonsynonymous mutations, a threshold that is predictive for potential benefit from ICIs (19). With this encouraging data, as well as prior gastric cancer studies that have shown benefit from cellular adoptive immunotherapy (20), ICIs have been positioned as new potential therapeutic strategies.
Initial studies of CTLA-4 antagonism in gastric and GEJ cancer failed to demonstrate significant clinical benefit. In a phase II trial of the CTLA-4 inhibitor tremelimumab at doses of 15 mg/kg every 90 days, 18 previously-treated patients with metastatic gastric or esophageal adenocarcinoma were enrolled (21). Clinical benefit was minimal with stable disease and subjective clinical improvement noted in four patients and a partial response in one patient after 25 months on treatment. Median OS was 4.83 months and median time to progression (TTP) was 2.83 months, similar to other second-line treatments. A more recent randomized phase II trial comparing ipilimumab as a maintenance strategy (10 mg/kg every 3 weeks for 4 doses and then every 12 weeks for up to 3 years) to best supportive care after first-line chemotherapy in 114 patients with unresectable, locally advanced gastric or GEJ cancer, found no statistical difference in immune-related PFS or median OS between both groups (22).
While trials of CTLA-4 inhibitors have been unremarkable, studies of PD-1 antagonists have yielded more impressive results. A multi-center, open-label, phase Ib trial led by Muro et al. evaluated the safety profile and efficacy of pembrolizumab in a mostly pretreated population of patients with metastatic adenocarcinoma of the stomach or GEJ (23). Only patients with >1% PD-L1 expression in stroma or tumor cells on immunohistochemistry utilizing the 22C3 antibody were enrolled in the study. Overall, 39 patients received pembrolizumab intravenously at 10 mg/kg once every 2 weeks for a maximum of 24 months. Among 36 evaluable patients, 8 patients (22%) had a partial response by central review. No complete responses were observed. Overall, 17 (53%) of 32 patients with a post-baseline tumor assessment had a decrease in the size of their target lesions. Median time to response was 8 weeks and median duration of response was 40 weeks. In addition to a manageable toxicity profile, four of the eight responders were alive without disease progression or subsequent cancer therapy at last assessment. These favorable findings in patients with gastric and GEJ adenocarcinoma were similar to those reported in a phase Ib study of patients with mostly esophageal squamous cell carcinoma. In this study, 23 patients with PD-L1 positive advanced adenocarcinoma or squamous cell carcinoma of the esophagus were treated with pembrolizumab 10 mg/kg every 2 weeks for up to 2 years (24). ORR at data cut-off was 30.4% with a 6- and 12-month PFS rate of 30.4% and 21.7% respectively.
In addition to pembrolizumab, preliminary data of single-agent nivolumab in advanced gastric or GEJ cancer demonstrated comparable efficacy. Without regard to PD-L1 status, 59 previously treated patients received nivolumab 3 mg/kg every 2 weeks until disease progression or unmanageable toxicity (25). With minimal treatment-related adverse effects, ORR was 12% (1 complete, 6 partial responses), with a median duration of response of 7.1 months. In addition, median OS was 6.8 months; 12-month OS rate was 38%. Interestingly, the ORR in patients with PD-L1-positive and PD-L1-negative tumors was 18% and 12%, respectively. Furthermore, new data of PD-L1 inhibitor avelumab as first-line maintenance or second-line therapy in gastric and GEJ cancer showed promising activity in 151 unselected patients (26). Administered at 10 mg/kg every 2 weeks, avelumab led to a clinical response in 9.7% of those in the second-line therapy cohort (all partial responses) and 9.0% of those in the first-line cohort (2 complete, 6 partial responses). Disease control rate was 29.0% and 57.3%, and median PFS was 6.0 and 12.0 weeks in second-line and first-line maintenance uses respectively.
While many clinical trials of ICIs in gastric and gastroesophageal cancer are ongoing (see Table 1), preliminary data support the efficacy of PD-1/PD-L1 inhibitors in this disease state, especially when PD-L1 expression is over 1%. It will be important to continue to evaluate the role of ICI predictive biomarkers, including PD-L1, overall tumor mutational load and unique gastric cancer immune signatures. In addition, more data regarding combination PD-1 blockade and additional agents may be useful in assessing optimal clinical responsiveness.
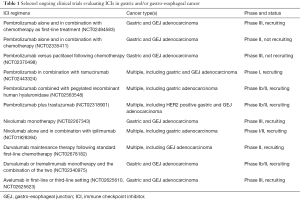
Full table
Colorectal cancer (CRC)
Despite a dismal 5-year survival rate for patients with metastatic CRC on current standard-of-care regimens, advances in our understanding of the CRC tumor genome have elucidated the complex molecular landscape of the disease and the prospects of more personalized treatment strategies (27). About 15% of CRCs are hyper-mutated due to deficient mismatch repair (dMMR) proteins and resulting MSI-high. Alternatively, the majority of CRCs have proficient mismatch repair (pMMR) proteins, are microsatellite stable (MSS), and are not hypermutated (28). Clinical trials of ICI therapy in both these CRC subtypes have revealed unique results depending on MSI tumor status. An initial study of 45 treatment-refractory CRC patients with unknown MSI status given single-agent tremelimumab did not clinically improve with this therapy (29). In phase I studies of PD-1 (30) and PD-L1 (31) antagonists in 19 and 18 unselected CRC patients respectively, there were no documented responses. A similar phase I trial of nivolumab in 14 patients with CRC showed minimal efficacy (32), yet 3-year follow-up data from this phase I trial found one CRC patient with MSI-high who was treated with 5 doses over 19 months and achieved a complete response lasting more than 3 years (33), prompting further investigation into whether this subgroup could potentially benefit from ICIs.
Patients with dMMR and resultant MSI-high tumors have a vast accumulation of frameshift and missense mutations in “microsatellite” trinucleotide repeat sequences that can potentially make them immunologically susceptible to immune-activating treatment such as ICIs (34). The high mutational load of these tumors may facilitate the formation of tumor-specific neoantigens, which can trigger a more robust immune response driven by tumor-infiltrating lymphocytes (TILs) (35). This is supported by data suggesting a direct correlation between the CD8+ TIL density and total number of frameshift mutations as well as in vitro activation of cytotoxic T-cells by peptides derived from frameshift mutations (36). In addition, recent data suggest both mutational burden and TIL infiltration are potential predictive biomarkers of ICI efficacy (37). Moreover, MSI-high CRC should be further amenable to PD-1 directed therapy given the significantly higher rates of PD-1 and PD-L1 expression in dMMR compared to pMMR tumors (38) and the role of PD-L1 as an additional predictive biomarker of PD-1 blockade (37).
An important phase II study by Le et al. evaluated the clinical activity of PD-1 inhibitor pembrolizumab in patients with treatment-refractory metastatic CRC with or without MMR deficiency (39). A total of 32 patients with CRC (10 with dMMR and 18 with pMMR) were given 10 mg/kg of pembrolizumab every 14 days. Patients with dMMR CRC achieved greater immune-related ORR (40%) and PFS at 20 weeks (78%) than patients with pMMR CRC (0% and 11% respectively). In addition, patients with dMMR CRC had a median PFS and OS that was not reached, while for patients with pMMR tumors, the numbers were 2.2 and 5.0 months respectively. A recent update with an expanded cohort presented at ASCO 2016 corroborated the impressive clinical benefit of pembrolizumab in the dMMR CRC subgroup (40). A total of 53 CRC patients (28 dMMR and 25 pMMR) were evaluated, and a 50% ORR was identified in the dMMR cohort with no responses in the pMMR cohort. Median PFS and median OS were not reached for the dMMR subgroup, but were 2.4 and 6 months, respectively, for the pMMR subgroup.
Preliminary data from a phase Ib study also presented at ASCO 2016 was the first to demonstrate clinically relevant activity of PD-1 blockage in pMMR CRCs. This trial evaluated the combination of PD-L1 inhibitor atezolizumab (Tecentriq®, Genentech, San Francisco, USA) and MEK inhibitor cobimetinib (Cotellic®, Genentech) in patients with heavily pre-treated pMMR metastatic CRC (41). Escalating doses of cobimetinib were given in conjunction with 800 mg of atezolizumab every 2 weeks in 23 patients. MEK inhibition was found to induce PD-L1 expression and was able to elicit immune activity in pMMR CRCs. The ORR was 17%, with tumor shrinkage of at least 30% in 4 patients and stable disease in 5 patients (22%). Of the four responders, three had CRCs that were pMMR and one had a tumor with an unknown MMR status. Responses were ongoing at the data cut-off point and lasted up to 7.7 months. As this study suggests, combination therapies with ICI can be exploited to enhance immune activity against cancer, and this is especially important in pMMR tumors that have not benefited from the ICI revolution of cancer care.
While more data must accrue for better evaluation of the role of ICIs in metastatic CRC (ongoing studies in Table 2), preliminary data suggest that PD-1 inhibitors are efficacious in dMMR metastatic CRC. However, given the fact that only up to 15% of metastatic CRCs are dMMR genotype (42), novel strategies to prime the immune system to generate a response in pMMR CRC tumors are warranted. Preliminary data support the immune-priming effect of MEK inhibition in this regard. With more clinical data to be available in the future, the role of ICIs in CRC will be more fully defined.
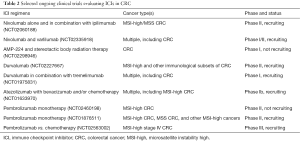
Full table
Pancreatic cancer
With inherent genetic instability, prominent desmoplastic stromal features and an immunosuppressive tumor microenvironment characterized by myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg), pancreatic ductal adenocarcinoma (PDAC) has a unique histopathologic and molecular makeup that makes it resistant to conventional chemotherapeutic regimens (43). Novel strategies employing ICIs have been of interest given the potential to combat the tumor’s predilection for immune evasion and reverse the immunosuppressive stromal microenvironment.
Early clinical trials of ICIs in PDAC did not produce the much anticipated promising results. An early phase I study of the PD-L1 inhibitor BMS-936559 found no radiographic responses in 14 patients with pancreatic cancer (31). Furthermore, a phase II trial of ipilimumab monotherapy in 27 previously treated patients with metastatic and locally advanced PDAC was halted prematurely after there were no identified responders by RECIST criteria (44). In this study, patients were given intravenous doses of ipilimumab every 3 weeks at 3 mg/kg for a maximum of two courses (4 doses per course). Only 8 of 20 patients with metastatic disease were able to finish a single course due to progressive disease. Nonetheless, one patient with metastatic PDAC to the liver did experience a delayed resolution of initial progressive disease after 2 doses of ipilimumab, suggesting some patients may potentially derive protracted benefit from ICIs.
A follow-up phase Ib open-label study randomized 30 patients with previously treated advanced PDAC to either ipilimumab 10 mg/kg alone or ipilimumab 10 mg/kg in combination with a granulocyte-macrophage colony-stimulating factor (GM-CSF)-based whole-cell vaccine (GVAX) (45). Doses were administered every 3 weeks for a total of 4 doses followed by maintenance dosing every 12 weeks. Study findings were notable for an increased OS in patients in the combination therapy arm in comparison to those in the ipilimumab monotherapy arm (median OS 5.7 vs. 3.6 months and 12-month OS 27% and 7%, respectively). Similar immune-related adverse events were found in both arms and comparable to prior studies. Results from this study show improved efficacy from combination ICIs and an active T-cell inducing agent, supporting strategies in PDAC to alter the innate tumor microenvironment in conjunction with blocking immunoregulatory signals.
Another trial employed the GVAX vaccine in combination with CRS-207, a recombinant live-attenuated Listeria monocytogenes designed to secrete mesothelin into the cytosol of infected antigen presenting cells (46). GVAX has been shown to induce T cells against pancreas cancer antigens, including mesothelin-specific T cell responses. A randomized phase II trial assigned 93 previously treated patients with metastatic pancreas cancer in a 2:1 fashion to GVAX alone versus GVAX plus CRS-207 with a primary endpoint of OS. Approximately half of these patients had two or more lines of therapy and about 20% had lung only metastatic disease. The data monitoring committee determined that this trial met its prespecified criteria for early stopping and patients were crossed-over to the combination arm. The interim survival analysis for the intention-to-treat population revealed a median OS of 6 versus 3.4 months (one-sided P value of 0.0057) in the GVAX/CRS-207 versus GVAX alone arms, respectively. Surprisingly, the phase IIb trial reported the combination to be ineffective (47). An alternative vaccine strategy exploiting dendritic cells was assessed in a pilot study in combination with low-dose nivolumab. Of seven patients with stage IV pancreatic cancer enrolled, two patients had a partial remission (individual OS of 13 and 5 months, respectively, at the time of data cutoff) (48). Both patients are still alive with an ongoing therapeutic response, suggesting that innovative forms of PD-1 inhibitors and vaccines may prove useful in pancreatic cancer.
In addition to vaccines, strategies to employ ICIs and chemotherapy have been attempted with evidence of efficacy and a tolerable safety profile in patients with pancreatic cancer. Preliminary data from a phase Ib study of combination ipilimumab and gemcitabine was presented at ASCO 2016 (49). Sixteen patients were enrolled with 13 at dose-escalation and 3 at maximum-tolerated dose. Partial remission and stable disease by waterfall plot were seen in 43% of patients and 38% had progressive disease. Median PFS was 2.5 months and median OS was 8.5 months.
PD-1 inhibitors have also been tested in subsets of patients with PDAC, specifically those with MSI-high disease. Though this population makes up only around 3% of PDAC patients (50), it does appear that dMMR in this subset may increase susceptibility to PD-1 inhibitors. In a phase II study of pembrolizumab (10 mg/kg every 2 weeks) in 17 previously-treated patients with a variety of advanced dMMR non-CRCs (including 4 ampullary, 4 pancreas, 3 biliary, 3 small bowel and 3 gastric) ORR was 50%, disease control rate 70% and OS 21 months in 10 evaluable patients at time of analysis (40).
Though initial single-agent studies of ICIs demonstrated little benefit in patients with pancreatic cancer, new understanding of the immunologic mechanisms in the tumor microenvironment of pancreatic cancer has enabled more successful synergistic strategies to overcome the stromal barrier. Ongoing studies are listed in Table 3. Just as MSI-high tumors have been shown amenable to the effects of ICIs, sensitizing the immune system with vaccines and/or additional chemotherapeutic agents has been shown to be a new therapeutic avenue with increasing clinical relevance.
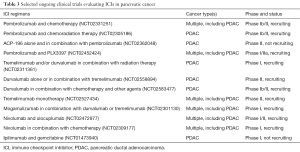
Full table
Biliary cancer
Detailed molecular characterization of patients with biliary tract carcinoma has revealed that a subset of patients with advanced disease have increased expression of immune checkpoint molecules (i.e., PD-L1, CTLA-4), increased total number of somatic mutations, and a hypermutated genotype that may be particularly responsive to ICI therapy (51). While positive data has accrued with pembrolizumab in patients with dMMR biliary cancer as previously reported (50), an additional trial of pembrolizumab in patients with advanced biliary tract carcinoma has implicated the potential for PD-1 efficacy in this broader cohort as well. A phase Ib, multi-cohort trial of pembrolizumab 10 mg/kg every 2 weeks (for up to 2 years) was conducted in 24 heavily pre-treated patients with PD-L1 positive adenocarcinoma of the gallbladder or biliary tree (52). PD-L1 positivity was defined as greater than 1% PD-L1 tumor expression by a prototype immunohistochemical assay. Overall, interim results revealed an ORR of 17.4% (4 partial responses) and stable disease in 17.4%. Median duration of response was not reached and the therapy was well-tolerated.
Altogether, the limited data in biliary cancer suggest efficacy of PD-1 inhibition, with some durable responses noted. New clinical trials with novel combinations of pembrolizumab plus induction GM-CSF (NCT02703714) and pembrolizumab plus ramucirumab (NCT02443324) are underway. As results from these and other clinical trials accumulate, the role of ICIs in the management of this rare cancer will be more fully elucidated.
Anal cancer
Though an uncommon malignancy, the incidence of squamous cell carcinoma of the anal canal (SCCA) is on the gradual rise (53). Current treatment of this human papillomavirus-related cancer centers on chemoradiotherapy with fluorouracil and mitomycin C for localized disease, though metastatic disease lacks a consensus approach to treatment (54). A recent study of ICIs in the treatment of metastatic SCCA was recently presented at the 2016 World Congress on GI cancer with encouraging results. In this phase II trial, 37 previously treated patients were administered nivolumab at 3 mg/kg IV every 2 weeks (55). Overall, ORR was 24.3% (2 complete, 7 partial responses) and stable disease was achieved in 17 patients. Median PFS was 3.9 months and nivolumab was well-tolerated in this population. This study supports the continued evaluation of ICIs in this tumor type and the potential for efficacy in a virally-mediated, immune evasive disease.
Conclusions
In contrast to the early demonstrated efficacy of ICIs in melanoma and NSCLC, the role these novel immunomodulating agents play in GI cancers has only recently been appreciated. A more sophisticated understanding of the genomic and molecular landscape of specific GI tumors has elucidated potential mechanisms for enhanced checkpoint response, including increased PD-L1 expression, elevated mutational burden, and an inflammatory intra-tumoral milieu. Though single-agent ICI treatment had been largely ineffective initially, encouraging results in MSI-high tumors and PD-L1 positive gastric and gastroesophageal cancer suggest that ICIs are a promising treatment strategy in these subgroups of patients. In addition to small studies demonstrating response to PD-1 inhibition in biliary and SCCA, preliminary data of combination regimens in pMMR CRC and pancreatic cancer have shown early efficacy. With numerous prospective clinical trials of ICIs in GI tumors still underway, this therapeutic approach promises to be an exciting new option for many patients with recalcitrant GI malignancies, yet optimization of combination strategies and validation of predictive biomarkers are needed to ensure the most robust anti-tumor response.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Marin Feldman Xavier) for the series “Advances on Clinical Immunotherapy” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.11). The series “Advances on Clinical Immunotherapy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pennock GK, Chow LQ. The Evolving Role of Immune Checkpoint Inhibitors in Cancer Treatment. Oncologist 2015;20:812-22. [Crossref] [PubMed]
- Kohrt HE, Tumeh PC, Benson D, et al. Immunodynamics: a cancer immunotherapy trials network review of immune monitoring in immuno-oncology clinical trials. J Immunother Cancer 2016;4:15. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [Crossref] [PubMed]
- Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. [Crossref] [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Santabarbara G, Maione P, Rossi A, et al. Novel immunotherapy in the treatment of advanced non-small cell lung cancer. Expert Rev Clin Pharmacol 2016;9:1571-81. [Crossref] [PubMed]
- Harris SJ, Brown J, Lopez J, et al. Immuno-oncology combinations: raising the tail of the survival curve. Cancer Biol Med 2016;13:171-93. [Crossref] [PubMed]
- Jia ZF, Zhang SL, Cao XY, et al. Interaction between Helicobacter pylori and host genetic variants in gastric carcinogenesis. Future Oncol 2016;12:2127-34. [Crossref] [PubMed]
- Abdel-Latif MM, Duggan S, Reynolds JV, et al. Inflammation and esophageal carcinogenesis. Curr Opin Pharmacol 2009;9:396-404. [Crossref] [PubMed]
- Raufi AG, Klempner SJ. Immunotherapy for advanced gastric and esophageal cancer: preclinical rationale and ongoing clinical investigations. J Gastrointest Oncol 2015;6:561-9. [PubMed]
- Festino L, Botti G, Lorigan P, et al. Cancer Treatment with Anti-PD-1/PD-L1 Agents: Is PD-L1 Expression a Biomarker for Patient Selection? Drugs 2016;76:925-45. [Crossref] [PubMed]
- Colli LM, Machiela MJ, Myers TA, et al. Burden of Nonsynonymous Mutations among TCGA Cancers and Candidate Immune Checkpoint Inhibitor Responses. Cancer Res 2016;76:3767-72. [Crossref] [PubMed]
- Jiang JT, Shen YP, Wu CP, et al. Increasing the frequency of CIK cells adoptive immunotherapy may decrease risk of death in gastric cancer patients. World J Gastroenterol 2010;16:6155-62. [Crossref] [PubMed]
- Ralph C, Elkord E, Burt DJ, et al. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res 2010;16:1662-72. [Crossref] [PubMed]
- Moehler MH, Cho JY, Kim YH, et al. A randomized, open-label, two-arm phase II trial comparing the efficacy of sequential ipilimumab (ipi) versus best supportive care (BSC) following first-line (1L) chemotherapy in patients with unresectable, locally advanced/metastatic (A/M) gastric or gastro-esophageal junction (G/GEJ) cancer. J Clin Oncol 2016;34:abstr 4011.
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. [Crossref] [PubMed]
- Doi T, Piha-Paul SA, Jalal SI, et al. Updated results for the advanced esophageal carcinoma cohort of the phase Ib KEYNOTE-028 study of pembrolizumab (MK-3475). J Clin Oncol 2016;34:abstr 7.
- Le DT, Bendell JC, Calvo E, et al. Safety and activity of nivolumab monotherapy in advanced and metastatic (A/M) gastric or gastroesophageal junction cancer (GC/GEC): Results from the CheckMate-032 study. J Clin Oncol 2016;34:abstr 6.
- Chung HC, Arkenau HT, Wyrwicz L, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced gastric or gastroesophageal junction cancer from JAVELIN solid tumor phase Ib trial: Analysis of safety and clinical activity. J Clin Oncol 2016;34:abstr 4009.
- Sun X, Suo J, Yan J. Immunotherapy in human colorectal cancer: Challenges and prospective. World J Gastroenterol 2016;22:6362-72. [Crossref] [PubMed]
- Müller MF, Ibrahim AE, Arends MJ. Molecular pathological classification of colorectal cancer. Virchows Arch 2016;469:125-34. [Crossref] [PubMed]
- Chung KY, Gore I, Fong L, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol 2010;28:3485-90. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. [Crossref] [PubMed]
- Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res 2013;19:462-8. [Crossref] [PubMed]
- Sinicrope FA, Sargent DJ. Molecular pathways: microsatellite instability in colorectal cancer: prognostic, predictive, and therapeutic implications. Clin Cancer Res 2012;18:1506-12. [Crossref] [PubMed]
- Deschoolmeester V, Baay M, Van Marck E, et al. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol 2010;11:19. [Crossref] [PubMed]
- Maby P, Tougeron D, Hamieh M, et al. Correlation between Density of CD8+ T-cell Infiltrate in Microsatellite Unstable Colorectal Cancers and Frameshift Mutations: A Rationale for Personalized Immunotherapy. Cancer Res 2015;75:3446-55. [Crossref] [PubMed]
- Hermel D, Sigal D. “Check”-ing the data: a review of immune checkpoint inhibitor biomarkers. Personalized Medicine in Oncology 2016;5:234-40.
- Lee LH, Cavalcanti MS, Segal NH, et al. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol 2016;29:1433-42. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in mismatch repair deficient non-colorectal gastrointestinal cancers. J Clin Oncol 2016;34:abstr 195.
- Bendell JC, Kim TW, Goh BC, et al. Clinical activity and safety of cobimetinib (cobi) and atezolizumab in colorectal cancer (CRC). J Clin Oncol 2016;34:abstr 3502.
- Goldstein J, Tran B, Ensor J, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann Oncol 2014;25:1032-8. [Crossref] [PubMed]
- Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 2007;67:9518-27. [Crossref] [PubMed]
- Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010;33:828-33. [Crossref] [PubMed]
- Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 2013;36:382-9. [Crossref] [PubMed]
- Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015;33:1325-33. [Crossref] [PubMed]
- Aduro Biotech. Press Release. Aduro Biotech Announces Phase 2b ECLIPSE Trial Misses Primary Endpoint in Heavily Pretreated Metastatic Pancreatic Cancer. Available online: investors.aduro.com/phoenix.zhtml?c=242043&p=irol-newsArticle&ID=2168543
- Nesselhut J, Marx D, Lange H, et al. Systemic treatment with anti-PD-1 antibody nivolumab in combination with vaccine therapy in advanced pancreatic cancer. J Clin Oncol 2016;34:abstr 3092.
- Kalyan A, Kircher SM, Mohindra NA, et al. Ipilimumab and gemcitabine for advanced pancreas cancer: A phase Ib study. J Clin Oncol 2016;34:abstr e15747.
- Williams AS, Huang WY. The analysis of microsatellite instability in extracolonic gastrointestinal malignancy. Pathology 2013;45:540-52. [Crossref] [PubMed]
- Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015;47:1003-10. [Crossref] [PubMed]
- Bang YJ, Doi T, De Braud F, et al. Safety and efficacy of pembrolizumab (MK-3475) in patients (pts) with advanced biliary tract cancer: Interim results of KEYNOTE-028. Eur J Cancer 2015;51:S112. [Crossref]
- van der Zee RP, Richel O, de Vries HJ, et al. The increasing incidence of anal cancer: can it be explained by trends in risk groups? Neth J Med 2013;71:401-11. [PubMed]
- Bernardi MP, Ngan SY, Michael M, et al. Molecular biology of anal squamous cell carcinoma: implications for future research and clinical intervention. Lancet Oncol 2015;16:e611-21. [Crossref] [PubMed]
- Morris V, Ciombor K, Salem M, et al. A multi-institutional phase II study of single agent nivolumab in previously treated metastatic squamous cell carcinoma of the anal canal (SCCA). 2016 World Congress on GI Cancer. Barcelona, Spain, June 28 - July 2, 2016.


