
The prospective role of matrix metalloproteinase-2/9 and transforming growth factor beta 1 in accelerating the progression of hepatocellular carcinoma
Hepatocellular carcinoma (HCC) related to cirrhosis is one of the most important health issues worldwide. The pathogenesis of cirrhosis is a result of chronic liver disease (CLD), which is characterized by an excessive accumulation of extracellular matrix (ECM) protein. The matrix metalloproteinases (MMPs) such as MMP-1, 2, 8, 9 and transforming growth factor beta 1 (TGF-β1) play important roles in the progression in CLD and HCC (1,2). The comment of Dr. Feitelson on our paper of which the title was “Reciprocal activation between MMP-8 and TGF-β1 stimulates EMT and malignant progression of hepatocellular carcinoma” in Cancer Letters (3) highlighted that the reciprocal activation between MMP-8 and TGF-β1 could be the target to delay or prevent the development of cirrhosis and/or HCC (4).
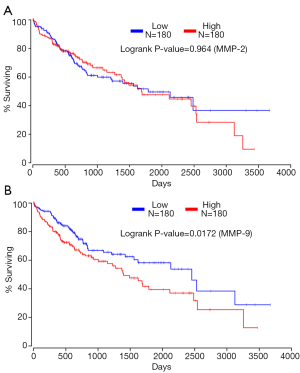
Besides MMP-8, many studies have indicated that the expression of MMP-2/9 is more likely to be upregulated in highly invasive HCC cells than that in the lowly invasive and non-invasive cells. Furthermore, the overexpression of MMP-2/9 has been correlated to the invasiveness and metastasis of HCC, and MMP-9 is superior to MMP-2 for the prediction of tumor recurrence and survival in HCC (5). Consistent with these studies, our previous data showed that the mRNA and protein levels of MMP-9 and MMP-2 were significantly higher in highly invasive SK-Hep-1 and HCCLM3 cells than that in lowly invasive HepG2 and SMMC-7721 cells and normal liver HL-7702 cells. Furthermore, knocking down TGF-β1 significantly decreased the mRNA and protein levels of MMP-9 and MMP-2 in SK-Hep-1 and HCCLM3 cells [Supplementary material in (3), which can be found online at http://dx.doi.org/10.1016/j.canlet.2016.02.001]. In addition, TGF-β1 has been proved to be an independent indictor for the survival in HCC (3). Herein, the high throughput RNA-seq data from The Cancer Genome Atlas (TCGA) suggested that high expression of MMP-9 was also closely related with poor survival rate (Figure 1A, n=360), which showed a similar predictive value as TGF-β1. However, there is no significant difference in survival between HCC tissues with high and low MMP-2 expression (Figure 1B, n=360), which suggests that MMP-9 could be more sensitive than MMP-2 for the prediction of HCC patients’ survival. Therefore, our previous results indicate that genetic targeting-modification of TGF-β1 can affect the expression levels of MMP-2 and MMP-9 in HCC cells, suggesting that TGF-β1 may similarly regulate some other members of the MMP family besides MMP-8. Some in vitro studies also pointed out the consistent relationship between MMP-2/9 and TGF-β1. In several cancer cells or some fibroblasts, TGF-β1 may also upregulate MMP-2 and MMP-9 expression via direct activation of transcription factors or several signal pathways, thus promoting the progression of tumor proliferation (6). Furthermore, TGF-β1, especially in HCC, has been shown to induce the expression of MMP-2 and MMP-9, which can promote the migratory/invasive capacities of HCC cells (7). The link between MMP-2/9 and TGF-β1 can also be explained from other studies that, MMP-2 and MMP-9 drive stroma-mediated invasion and metastasis via cleaving the latency associated peptide (LAP) of TGF-β1 to increase TGF-β1 bioactivity. In addition, Okamoto et al. indicated that functional gene polymorphisms of MMP-1, MMP-3 and MMP-9 were associated with the progression of CLD (8), suggesting that there is a potential positive relationship between MMP-2 or MMP-9 and TGF-β1. Finally, the interaction of MMPs and TGF-β1 forms a bidirectional regulatory feedback loop associated with liver fibrosis and HCC, and the clinical significance of MMP-9 in HCC prognosis and liver fibrosis is superior to that of MMP-2. However, the exact effect and potential molecular mechanism of this loop on CLD or HCC has not been clarified yet. The clinical significance of MMP-2 and MMP-9 in CLD and HCC still need to be investigated with a larger number of samples involved.
The pathogenesis of liver fibrosis is the excessive accumulation and deposition of ECM proteins, and the subsequent progression toward HCC has been well established. The hepatic stellate cells (HSCs) and epithelial–mesenchymal transition (EMT) have been reported to be involved in the progression of CLD and HCC (6), and several MMP members and TGF-β1 are the inductor of HSCs and EMT. For example, TGF-β1 plays a key role in the progression of CLD, in which it induces fibrogenesis through activation of the HSCs, therefore inducing the progression of HCC. Besides, the TGF-β1-activated Smad-signaling pathway has been considered as a potential target for cirrhosis therapy, for it can stimulate the synthesis of ECM-associated proteins, and inhibit their degradation (9). Furthermore, other ECM-degradation associated factors such as type IV collagen, fibrinogen, and urokinase type plasminogen activator stimulate HSCs via activating latent cytokines of TGF-β1 (9). In addition, MMPs are the major group of proteinases to regulate the ECM remodeling, so they are essential in the process of liver fibrosis or HCC. It has been reported that circulating MMP-2 and MMP-9 levels increased significantly in cirrhosis so it can become a potential diagnostic marker of liver fibrosis in patients with chronic hepatitis C (10). In HCC cells, MMP-2, MMP-8, MMP-9 and TGF-β1 can induce EMT and promote the malignant progression of HCC (6). These studies indicate that TGF-β1 and MMPs, such as MMP-2 and MMP-9 can promote the progression of CLD or HCC via stimulating HSCs or EMT. Particularly, platelet-derived growth factor (PDGF)-BB and TGF-β1 promote the migration of HSCs and CLD via MMP-2/9-dependent mechanism (11).
In conclusion, we do agree with the insightful idea of Dr. Feitelson and we can imply that the potential regulatory feedback loop between MMP-8 and TGF-β1, as well as between MMP-2/9 and TGF-β1, may promote CLD progression to HCC. The research that we have conducted has its limitations though. Consequently, both in vitro and in vivo experiments are needed to verify the prospective role of MMP-2/9 and TGF-β1 in the progression of CLD and HCC.
Acknowledgments
Funding: This work was supported by the Science Foundation for Youths of Guangxi Province (2012GXNSFBA053096).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Xiao-Yu Yang (Department of special treatment II at Eastern Hepatobiliary Surgery Hospital, the Third Affiliated Hospital, Second Military Medical University (SMMU), Shanghai, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.02.41). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Giannelli G, Mikulits W, Dooley S, et al. The rationale for targeting TGF-β in chronic liver diseases. Eur J Clin Invest 2016;46:349-61. [Crossref] [PubMed]
- Zeng Y, Yao X, Chen L, et al. Sphingosine-1-phosphate induced epithelial-mesenchymal transition of hepatocellular carcinoma via an MMP-7/ syndecan-1/TGF-β autocrine loop. Oncotarget 2016;7:63324-37. [PubMed]
- Qin G, Luo M, Chen J, et al. Reciprocal activation between MMP-8 and TGF-β1 stimulates EMT and malignant progression of hepatocellular carcinoma. Cancer Lett 2016;374:85-95. [Crossref] [PubMed]
- Feitelson MA. Can we stop the progression of chronic liver disease to hepatocellular carcinoma? Transl Cancer Res 2016;5:S794-9. [Crossref]
- Chen R, Cui J, Xu C, et al. The significance of MMP-9 over MMP-2 in HCC invasiveness and recurrence of hepatocellular carcinoma after curative resection. Ann Surg Oncol 2012;19:S375-84. [Crossref] [PubMed]
- Krstic J, Santibanez JF. Transforming Growth Factor-Beta and Matrix Metalloproteinases Functional Interplay in Cancer; Implications in Epithelial to Mesenchymal Transition. Cell Biol 2014;Res Ther S1:004.
- Wang B, Hsu SH, Majumder S, et al. TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene 2010;29:1787-97. [Crossref] [PubMed]
- Okamoto K, Mimura K, Murawaki Y, et al. Association of functional gene polymorphisms of matrix metalloproteinase (MMP)-1, MMP-3 and MMP-9 with the progression of chronic liver disease. J Gastroenterol Hepatol 2005;20:1102-8. [Crossref] [PubMed]
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209-18. [Crossref] [PubMed]
- Leroy V, Monier F, Bottari S, et al. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am J Gastroenterol 2004;99:271-9. [Crossref] [PubMed]
- Yang C, Zeisberg M, Mosterman B, et al. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology 2003;124:147-59. [Crossref] [PubMed]


