
A crowded, but still varied, space: brigatinib in anaplastic lymphoma kinase-rearranged non-small cell lung cancer
Considering that, as recently as 10 years ago, physicians caring for patients with advanced lung cancer had only a handful of conventional cytotoxic agents from which to choose, the field’s recent development and competition seem truly remarkable. A prime example of this shifting and crowded landscape is immunotherapy. Since 2015, three different checkpoint inhibitors targeting programmed death 1 (PD-1) or PD-1 ligand (PD-L1) have received U.S. FDA approval, with several others currently in clinical trials. While these drugs may differ by specific target (PD-1 versus PD-L1), antibody species (humanized versus fully human), and IgG subclass (IgG1 versus IgG4), it remains unclear whether there are clinically meaningful differences in efficacy or toxicity between these agents.
The treatment of anaplastic lymphoma kinase (ALK)-positive lung cancer has seen similar developments. Although these cases represent only 3–5% of non-small cell lung cancer (NSCLC), researchers and pharmaceutical companies have devoted intense effort to this disease subset. The field received an initial boost by the rapidity of drug development. Largely because the first-generation ALK inhibitor crizotinib was already under clinical development as a MET inhibitor, the interval between discovery of the ALK target and evidence of a clinically effective drug was a remarkably short 3 years (1-4), compared to 41 years between the discovery of BCR-ABL and approval of imatinib and 26 years between the discovery of epidermal growth factor receptor (EGFR) and approval of erlotinib (4). For ALK-positive lung cancer, the pace of development has not slowed. Within 3–5 years, so-called second-generation ALK inhibitors such as ceritinib and alectinib, both of which have clear activity in crizotinib-resistant cases, were available. By contrast, it took more than a decade to develop and approve a late-generation EGFR inhibitor that had meaningful efficacy in erlotinib- and gefitinib-resistant cases (5).
This time period also saw increased understanding of the heterogeneous and complex science of crizotinib resistance in ALK-positive lung cancer. Broadly, mechanisms can be characterized as pharmacologic or biologic. Pharmacologic reasons may include patient non-adherence, reduced absorption, drug interactions, and most importantly inadequate blood-brain barrier penetration. Indeed, up to 40% of progression on crizotinib occurs in the central nervous system (6). Biologic mechanisms include bypass tracks with alternate oncogenes such as EGFR and V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) (35% of cases) (7,8), ALK gene copy number gain (20% of cases) (9), and ALK resistance mutations (35% of cases). To date, more than a dozen ALK resistance mutations have been identified, including gatekeepers analogous to T790M in EGFR mutant NSCLC (10) and T315I in chronic myeloid leukemia, which reduce crizotinib binding and enhance ATP affinity (8,11-14). A potential explanation for why this secondary mutational landscape is more complex than that of EGFR (which is dominated by exon 20 T790M) is that EGFR resistance mutations appear to convey a selective growth disadvantage (8,15) whereas ALK mutations may increase proliferation (8).
In general, later-generation ALK inhibitors demonstrate efficacy in crizotinib-resistant cases through a number of features, including enhanced ALK kinase inhibition (16,17), better activity against second-site mutated ALK, activity against other oncogenic targets, and improved blood-brain barrier penetration (18). In contrast to the numerous PD-1 and PD-L1 inhibitors, the various ALK inhibitors have some clear and clinically meaningful differences, including toxicity. With crizotinib, characteristic adverse effects may include visual changes, peripheral edema, renal dysfunction, and orthostatic hypotension (19). For ceritinib, diarrhea and transaminitis require dose modification in approximately two-thirds of cases (15). Alectinib causes constipation and creatine phosphokinase elevations (20).
Continuing this trend, in a recently published phase 1/2 trial, Gettinger and colleagues show that the potent oral ALK inhibitor brigatinib has comparable efficacy to other late-generation ALK inhibitors but a distinct toxicity profile (21). In preclinical models, brigatinib has a broader spectrum of activity than ceritinib and alectinib, including not only ALK resistance mutations but also ROS1 fusions and mutant EGFR (22). The trial enrolled a total of 137 patients in a phase 1 dose escalation cohort (N=66) and five disease- and molecularly-defined phase 2 cohorts (N=69). Although multiple molecular diagnostic techniques for diagnosis of ALK positivity, including next generation sequencing and ALK protein expression by immunohistochemistry (23), in addition to fluorescent in situ hybridization (FISH), are now widely accepted in this trial, enrollment into ALK cohorts required demonstration of ALK gene fusion by FISH. Treatment-related adverse events were predominantly grade 1–2 and included nausea, fatigue, and diarrhea. Grade 3–4 events included increased lipase concentration, hypertension, and most notably pulmonary toxicity, including a 4% rate of fatal events. Radiographically, these cases featured linear or ground glass opacities. In the phase 2 trial, two dosing regimens were initially studied: 90 mg orally daily and 180 mg orally daily. Due to the emergence of pulmonary toxicity within 48 hours of treatment initiation in the 180 mg cohort, the schedule was modified to include a 7-day lead-in of 90 mg daily. Overall, 14% of patients required dose reductions.
Brigatinib demonstrated an efficacy profile expected for contemporary late-generation ALK inhibitors. Among the eight crizotinib-naïve ALK-rearranged cases, all responded [median progression-free survival (PFS) not reached]. Response rate was 74% for crizotinib-treated cases (median PFS 14.5 months). Intracranial response rate was 50%. Median intracranial PFS was 15.6 months for all assessable patients and 22.3 months for assessable patients with no prior brain radiotherapy. Activity was also noted in ROS1-positive NSCLC, as well as other ALK-rearranged malignancies including inflammatory myofibroblastic tumor and neuroendocrine tumor. Despite encouraging preclinical data, only 5% of EGFR-mutant NSCLC cases had an objective response.
These results lead to as many questions as they answer. Are second-generation ALK inhibitors best used as initial treatment or following crizotinib failure? It would seem that reserving second-generation ALK inhibitors for post-crizotinib failure would yield the greatest overall period of disease control. However, first-line use of drugs such as ceritinib or alectinib may have greater PFS than the overall combined PFS when they follow crizotinib (18,24). Which cases of ALK-positive brain metastases may be treated medically, and which are best approached initially with resection or radiation therapy? In this trial, enrollment of previously untreated brain metastases was limited to those that were neurologically stable and not requiring escalating steroid doses or anticonvulsants. Could brigatinib use be extended to those patients with symptomatic intracranial disease? At what point should disease progression on an ALK inhibitor be addressed with a change in systemic therapy, and when can local treatments be employed to prolong disease control? Small series have demonstrated that surgical resection or stereotactic ablative radiation in cases of oligo-progression, with continuation of the initial systemic targeted therapy, may extend disease control for several months (25). Such an approach is particularly effective against intracranial progression, presumably because it may represent failure of drug delivery rather than emergence of systemic resistance. Perhaps the most relevant to the trial under discussion: which second-generation ALK inhibitor has greatest efficacy? Which has the least toxicity?
While it may be difficult to distinguish the clinical efficacy of the various PD-1/PD-L1 inhibitors from one another, it has become clear that there are sufficient distinguishing characteristics among ALK inhibitors that, at least in some cases, their selection may be tailored to individual cases (see Table 1). Importantly, non-ALK activity may differ substantially. Crizotinib and brigatinib have efficacy against ROS1-positive NSCLC, but alectinib does not. Unique among clinically available ALK inhibitors, crizotinib also has activity against NSCLC harboring tyrosine-protein kinase Met (cMET) exon 14 mutations (29,30). Activity also differs across the spectrum of secondary ALK resistance mutations. Indeed, some rare cases of molecular resistance to late-generation ALK inhibitors regain sensitivity to crizotinib (31). Realistically, there are too many mutations and too many drugs for clinicians to remember these associations. Awareness of and access to these data are critical to optimal patient care. Similarly, physicians must thoroughly understand each drug’s monitoring requirements and toxicity profile. Crizotinib may cause hypotension, while brigatinib may cause hypertension. Ceritinib may cause diarrhea, while alectinib may cause constipation. The visual changes associated with crizotinib may be striking. However, they do not impact visual acuity and resolve spontaneously in most cases despite continued drug administration. Oncologists unfamiliar with this clinical pattern may inappropriately reduce or discontinue dosing. Brigatinib pulmonary toxicity suggests that combinations with immune checkpoint inhibitors be approached with caution.
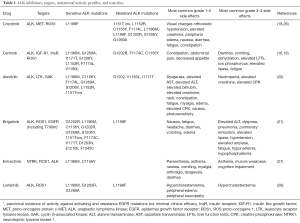
Full table
While the addition of brigatinib strengthens our anti-ALK armamentarium, it represents an incremental rather than revolutionary advance. ALK inhibitors and other molecularly targeted therapies requiring daily administration convey chronic toxicities that may rarely be severe but frequently impact quality of life. And clinical outcomes remain suboptimal. We continue to measure survival in intervals of several months. Particularly given the relatively young age of many ALK-positive patients cancer, in 2017 a diagnosis of advanced ALK-rearranged NSCLC remains tragic, with decades of life lost. Let us hope that forthcoming discoveries can truly change that.
Acknowledgments
Funding: This article is funded in part by a National Cancer Institute Midcareer Investigator Award in Patient-Oriented Research (K24CA201543-01) (to DE Gerber).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.02.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863-70. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol 2011;6:942-6. [Crossref] [PubMed]
- Gerber DE, Minna JD. ALK inhibition for non-small cell lung cancer: from discovery to therapy in record time. Cancer Cell 2010;18:548-51. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Sasaki H, Hikosaka Y, Kawano O, et al. Evaluation of Kras gene mutation and copy number gain in non-small cell lung cancer. J Thorac Oncol 2011;6:15-20. [Crossref] [PubMed]
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82. [Crossref] [PubMed]
- Katayama R, Khan TM, Benes C, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A 2011;108:7535-40. [Crossref] [PubMed]
- Shih JY, Gow CH, Yang PC. EGFR mutation conferring primary resistance to gefitinib in non-small-cell lung cancer. N Engl J Med 2005;353:207-8. [Crossref] [PubMed]
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 2012;4:120ra17 [Crossref] [PubMed]
- Sasaki T, Koivunen J, Ogino A, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res 2011;71:6051-60. [Crossref] [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [Crossref] [PubMed]
- Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734-9. [Crossref] [PubMed]
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22. [Crossref] [PubMed]
- Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 2014;4:662-73. [Crossref] [PubMed]
- Marsilje TH, Pei W, Chen B, et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem 2013;56:5675-90. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol 2016;17:1683-96. [Crossref] [PubMed]
- Zhang S, Anjum R, Squillace R, et al. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin Cancer Res 2016;22:5527-38. [Crossref] [PubMed]
- Conklin CM, Craddock KJ, Have C, et al. Immunohistochemistry is a reliable screening tool for identification of ALK rearrangement in non-small-cell lung carcinoma and is antibody dependent. J Thorac Oncol 2013;8:45-51. [Crossref] [PubMed]
- Rossi A. Alectinib for ALK-positive non-small-cell lung cancer. Expert Rev Clin Pharmacol 2016;9:1005-13. [Crossref] [PubMed]
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807-14. [Crossref] [PubMed]
- Heuckmann JM, Hölzel M, Sos ML, et al. ALK mutations conferring differential resistance to structurally diverse ALK inhibitors. Clin Cancer Res 2011;17:7394-401. [Crossref] [PubMed]
- Farago AF, Le LP, Zheng Z, et al. Durable Clinical Response to Entrectinib in NTRK1-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol 2015;10:1670-4. [Crossref] [PubMed]
- Infarinato NR, Park JH, Krytska K, et al. The ALK/ROS1 Inhibitor PF-06463922 Overcomes Primary Resistance to Crizotinib in ALK-Driven Neuroblastoma. Cancer Discov 2016;6:96-107. [Crossref] [PubMed]
- Ou SH, Greenbowe J, Khan ZU, et al. I1171 missense mutation (particularly I1171N) is a common resistance mutation in ALK-positive NSCLC patients who have progressive disease while on alectinib and is sensitive to ceritinib. Lung Cancer 2015;88:231-4. [Crossref] [PubMed]
- Toyokawa G, Inamasu E, Shimamatsu S, et al. Identification of a Novel ALK G1123S Mutation in a Patient with ALK-rearranged Non-small-cell Lung Cancer Exhibiting Resistance to Ceritinib. J Thorac Oncol 2015;10:e55-7. [Crossref] [PubMed]
- Shaw AT, Engelman JA. Crizotinib Resensitization by Compound Mutation. N Engl J Med 2016;374:1790-1. [Crossref] [PubMed]


