
Analysis of surgical treatment and prognostic factors for hepatocellular carcinoma with portal vein tumor thrombus
Introduction
Hepatocellular carcinoma (HCC) was the most common primary malignant liver tumor, which ranked in the third in China (1). The portal vein thrombosis (PVTT) of 10–40% were detected when HCC is exactly diagnosed (2,3). The patients whose PVTT existed in main branch of portal vein had poor prognosis. The survival time of symptomatic treatment was only 2–4 months (4). There were reports that hepatectomy could improve the survival rate of patients of HCC with PVTT (5). We retrospectively analyze the clinical and follow-up data to explore the prognosis and affecting factors of patients of HCC with PVTT after hepatectomy.
Methods
The clinical data
The clinical data of 81 patients of HCC with PVTT who were underwent surgical treatment were analyzed retrospectively between January 2000 and December 2013. Seventy-one cases were males and 10 cases were females. The mean age was 51.8 years (range, 31 to 73 years). All patients were followed up.
The treatments of postoperative and recurrence
Transcatheter arterial chemoembolization (TACE) on one month after surgery was underwent for patients with multiple lesions and macroscopic vascular tumor thrombosis and surgical margin less than 1.0 cm. Iodized oil were conventionally injected into HCC. It was positive that there were tumor staining during TACE or iodized oil was identified by CT scan on one month after TACE. The model of treatments for patients with recurrence was TACE and Sorafenib and hepatectomy and liver transplantation and symptomatic treatment, et al.
Follow-up
Follow-up was undergone in outpatient. It was once a month within 3 months after hepatectomy, and then once every three months. It was once every 6 months 2 years later. AFP and liver function and ultrasound and CT scan or MRI was performed when follow-up. Time of HCC recurrence or metastasis would be verified by imaging date.
Statistical
SPSS 13.0 software package was used for data analysis. Difference between groups was compared using chi-squared test. Survival analysis was analyzed using the Kaplan-Meier method (log rank test). The factor of P<0.05 was entered into the model of Cox’s proportional hazards regression.
Results
Survival analysis
The end point of follow-up time was ended at March 31, 2016 or death. The median time of follow-up was 11.0 months. The 1-, 2-, 3- and 5-year survival rates were 48%, 23%, 20% and 6%, respectively. The median overall survival (OS) time was 11.0 months. The 1-, 2-, 3- and 5-year disease-free survival (DFS) were 23%, 12%, 6% and 3%, respectively. The median DFS time was 4.2 months.
Affecting factors on the survival of patients of HCC with PVTT after hepatectomy
Kaplan-Meier analysis showed that surgical margins, blood loss, histological, Cheng’s classification for PVTT and TACE were important factors on the DFS of patients of HCC with PVTT (P<0.05). Cox’s proportional hazards regression showed that surgical margins and Cheng’s classification for PVTT were independent factors on the DFS (Table 1).
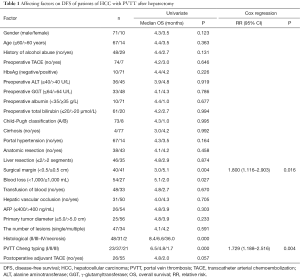
Full table
Affecting factors on OS time
The history of alcohol abuse and surgical margins and histological and Cheng’s classification for PVTT and treatment patterns for recurrence were important affecting factors on the DFS of patients of HCC with PVTT (P<0.05) (Table2). Cox regression risk model results showed histological and Cheng’s classification for PVTT and the model of treatment for recurrence were independent factors of survival (Figure 1).
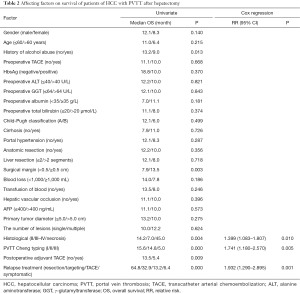
Full table
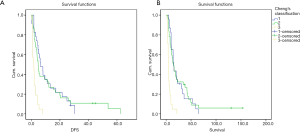
Subgroup analysis
Cheng’s classification for PVTT
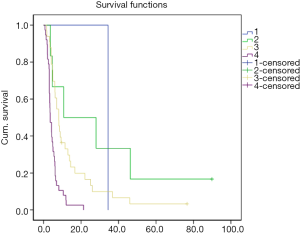
There were no patients with type IV of Cheng’s classification for PVTT in this group. There were no statistically significant difference of DFS and OS between type I and type II of patients. There were statistically significant difference of DFS and OS between type I and type III and type II and type III of patients.
Recurrence and treatment
HCC recurrence or metastasis were detected in 78 patients during follow-up. Intrahepatic HCC recurrence or metastasis were happened in 56 cases (71.8%) while extrahepatic HCC recurrence or metastasis were happened in 10 cases (12.8%). There were 12 cases (15.4%) who were found with intrahepatic and extrahepatic of HCC recurrence or metastasis, simultaneously. There were no statistically significant difference between groups (χ2=4.665, P=0.323). There were 26 cases (33.3%) with PVTT when recurrence or metastasis were conformed. There were no relationship with the Cheng’s classification for PVTT of the first liver resection (χ2=2.649, P=0.266).
Follow-up results showed that TACE was the mainly treatment for HCC recurrence. Single TACE was undergone in 33 patients (42.3%). Sorafenib was used in 6 patients (7.6%). Liver transplantation was performed in 1 patient. Symptomatic treatment was used in 38 patients (48.7%). Hepatectomy was performed in 1 case whose survival reached to 34.4 months. The survival rates of 2-year after recurrence for Sorafenib treatment and TACE and symptomatic treatment were 50.0%, 18.5% and 0%, respectively. The median survival time were 10.7, 8.0 and 3.5 months (log rank test: χ2=26.291, P=0.000, Figure 2). The relapse-free survival time of patients who took Sorafenib was significantly better than those of TACE and symptomatic treatment.
Discussion
Patients of HCC with PVTT had poor prognosis. The median survival time of symptomatic treatment was only 3.0 months (6). PVTT was also an independent risk factor of poor prognosis after hepatectomy (7). Shi et al. reported that 1-year, 3-year the OS and DFS of patients HCC with PVTT of after liver resection were 34%, 13% and 13%, 5%, respectively in one group of 406 cases (8). The cumulative survival rate of 1-, 2-, 3- and 5-year after liver resection were 48%, 23% and 20% respectively in this study. It could be related with different types of PVTT between two groups.
Prognosis was significantly correlated with the position of PVTT. Chen et al. (9) found that PVTT located in the main portal vein was a risk factor for one year recurrence by analyzing 438 patients of HCC with PVTT after hepatectomy. Compared with PVTT located in the branch of portal vein, its recurrence rate were 79% and 45%, and median survival time were 10.1 and 18.8 months, respectively. Li et al. (10) defined those types of PVTT depending on the location of PVTT in portal vein. Type I was PVTT located in the branch of grade two of portal vein and above. Type II was PVTT located in the branch of grade one of portal vein. Type III was PVTT located in the trunk of portal vein. Type IV was PVTT located in the superior mesenteric vein (SMV). Cheng’s classification for PVTT could objectively reflect the prognosis of patients of HCC with PVTT. With type of PVTT increasing, the survival time of patients decreased. According to Cheng’s classification for PVTT, type I, II and III were 23, 37 and 21 cases, respectively in our group. There were no statistically significant difference of DFS and OS between type I and type II of patients. The DFS and OS of type III were significantly shorter than type I and type II (P<0.05) which indicated that PVTT located in the main portal vein had poor prognosis.
Our previous results showed that PVTT was an important risk factor of short-term recurrence of liver resection (7). Therefore, comprehensive treatment after liver resection was very important. Multidisciplinary team (MDT) was one of the effective ways to improve the efficacy by multidisciplinary objective assessment, developing the best individualized treatment program and adjusting the treatment by observation (11). Yamamoto et al. (12) reported in a group of prognosis analysis of patients of HCC with PVTT that 5-year survival rate of patients of HCC with PVTT located in grade two, grade one and trunk of portal vein with TACE was higher than those without TACE, but there were no statistically significant difference between groups (29.3% vs. 11.3%, P=0.747). There were few patients with TACE before operation (7 cases) in our group. Compared to patients without TACE, there were no statistically significant difference on DFS and OS between two groups. There were 26 patients who received postoperatively adjuvant TACE. There was no significant difference between TACE group and without TACE group (4.8 and 2.0 months, P>0.05). Survival time of TACE group was significantly longer (13.5 and 5.4 months, P=0.009). The postoperative adjuvant TACE was not independent risk factor of prognosis. Peng et al. (13) reported in a group of 126 patients of HCC with PVTT after liver resection that the median OS of the TACE group and without TACE group were 13 and 10 months (P=0.0094), respectively. Adjuvant TACE could kill residual tumor and micro-metastases and increase the chances of DFS. It had synergistic effect with hepatectomy, which could improve long-term survival of patients of HCC with PVTT.
Li et al. (14) observed the influence of Sorafenib about 30 days on patients of HCC with PVTT after hepatectomy and found that the time to disease progression in patients of Sorafenib group (12 cases) was significantly longer (29 and 22 months, P=0.041) than in patients of without Sorafenib group. OS time between two groups was also significantly different (37 vs. 30 months, P=0.01). The results showed that all patients could tolerate sorafenib-related adverse effects by reducing sorafenib dose. Sorafenib could prolong survival time by inhibiting the growth of tumor after HCC recurrence. The study showed that the treatment mode of recurrence after hepatectomy for patients of HCC with PVTT was an independent risk factor of affecting prognosis. Two patients who relapsed received liver transplantation and liver resection, respectively. Survival time was 91.2 and 60.2 months. Six patients took sorafenib after recurrence (including 1 case for recurrence after liver transplantation). Survival time of sorafenib for recurrence was significantly better than of TACE and symptomatic treatment (28.2, 8.0 and 3.5 months; P<0.001). In the BCLC staging system and guideline, patients of HCC with PVTT was classified as advanced HCC and sorafenib was the standard treatment mode for the period (14). Sorafenib had anti-angiogenesis effect and anti-tumor activity, which possibly mediated by tyrosine kinase [including VEGF, PDGFRs, Raf and phosphatidylinositol 3-kinase/Akt/rapamycin (mTOR) signaling pathway targeting mammals] (15,16). Because sorafenib was expensive, few patients were entered into this group and there were only six cases in this group. It was about 11.7 months (1.3–32.2 months) from recurrence to taking sorafenib. All patients received TACE or radiotherapy (bone metastasis) during taking sorafenib, which indicated the effect of the MDT individualized therapy on extending survival.
All in all, Cheng’s classification for PVTT and treatment modal after recurrence were independent predictors of affecting survival of patients of HCC with PVTT. MDT individualized therapy based on the liver resection (including sorafenib) could effectively extend survival time of patients of HCC with PVTT.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2016.11.78). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethical committee, and written informed consent was obtained from each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Cheung TK, Lai CL, Wong BC, et al. Clinical features, biochemical parameters, and virological profiles of patients with hepatocellular carcinoma in Hong Kong. Aliment Pharmacol Ther 2006;24:573-83. [Crossref] [PubMed]
- Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62-7. [Crossref] [PubMed]
- Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol 2006;12:7561-7. [Crossref] [PubMed]
- Ban D, Shimada K, Yamamoto Y, et al. Efficacy of a hepatectomy and a tumor thrombectomy for hepatocellular carcinoma with tumor thrombus extending to the main portal vein. J Gastrointest Surg 2009;13:1921-8. [Crossref] [PubMed]
- Belli A, Cioffi L, Russo G, et al. Liver resection for hepatocellular carcinoma in patients with portal hypertension: the role of laparoscopy. Hepatobiliary Surg Nutr 2015;4:417-21. [PubMed]
- Levi Sandri GB, Colasanti M, Santoro R, et al. Laparoscopic right hepatectomy for hepatocellular carcinoma in cirrhotic patient. Hepatobiliary Surg Nutr 2015;4:436-8. [PubMed]
- Shi J, Lai EC, Li N, et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol 2010;17:2073-80. [Crossref] [PubMed]
- Chen XP, Qiu FZ, Wu ZD, et al. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol 2006;13:940-6. [Crossref] [PubMed]
- Li N, Wei XB, Cheng SQ. Application of cystoscope in surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. World J Gastroenterol 2016;22:5297-300. [Crossref] [PubMed]
- Li N, Feng S, Xue J, et al. Hepatocellular carcinoma with main portal vein tumor thrombus: a comparative study comparing hepatectomy with or without neoadjuvant radiotherapy. HPB (Oxford) 2016;18:549-56. [Crossref] [PubMed]
- Yamamoto Y, Ikoma H, Morimura R, et al. Post-hepatectomy survival in advanced hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol 2015;21:246-53. [Crossref] [PubMed]
- Peng BG, He Q, Li JP, et al. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg 2009;198:313-8. [Crossref] [PubMed]
- Li J, Hou Y, Cai XB, et al. Sorafenib after resection improves the outcome of BCLC stage C hepatocellular carcinoma. World J Gastroenterol 2016;22:4034-40. [Crossref] [PubMed]
- Bruix J, Sherman MAmerican Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Zhang CZ, Wang XD, Wang HW, et al. Sorafenib inhibits liver cancer growth by decreasing mTOR, AKT, and PI3K expression. J BUON 2015;20:218-22. [PubMed]


