
Checkpoint inhibitors in advanced lung cancer
Introduction
Lung cancer, while potentially curable in early stages, is often rapidly fatal once it becomes metastatic, and it continues to have the highest mortality rate of all cancers worldwide (1,2). Options other than cytotoxic chemotherapy were once limited. The identification of specific actionable mutations and development of corresponding targeted agents have offered non-chemotherapeutic treatment options in the metastatic setting for those tumors which have epidermal growth factor receptor (EGFR) mutations, or anaplastic lymphoma kinase (ALK) gene, and ROS proto-oncogene 1 receptor tyrosine kinase (ROS-1) gene rearrangements. Unfortunately, that still left many patients without non-cytotoxic treatment options. Progression after initial platinum-based chemotherapy for small cell lung cancer also has few effective treatment options, especially if there is platinum-resistance.
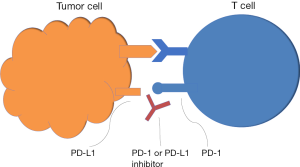
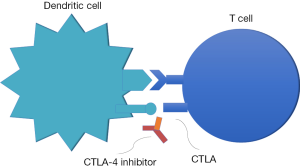
The identification of two immune cell interactions that down-regulate the activated T-cell’s ability to recognize and attack tumors has opened the door to new targets. The programmed cell death ligand-1 (PD-L1) and PD-L2 bind with the programmed cell death-1 (PD-1) checkpoint protein on T-cells which in turn, prevents the cytotoxic attack by the T-cells in the tumor microenvironment (3-5). Some tumors develop the ability to use this pathway in immune evasion (Figure 1). The second mechanism occurs at the level of the lymph nodes and involves the cytotoxic T-lymphocyte antigen 4 (CTLA-4) checkpoint protein expressed on T-cells binding to the receptor on dendritic cells, preventing the downstream priming of the T-cell to recognize cancer cells (6,7) (Figure 2). Antibodies developed to inhibit these checkpoint proteins restore the ability of the immune system to recognize and attack the cancer cells. See Table 1 for a list of agents reviewed.
Advanced Non-small cell lung cancer after platinum-based therapy
Pembrolizumab
KEYNOTE-001 was an international prospective phase I clinical trial with 5 cohorts and several expansion cohorts of patients with metastatic non-small cell lung cancer who were treatment naïve or had 1 or more prior lines of therapy. Patients were treated with single-agent pembrolizumab. Primary endpoints were dose-limiting toxicities and anti-tumor activity. A secondary objective was the validation of a companion diagnostic assay for the detection of PD-L1 expression by these tumors and determining a level of the expression that was predictive of response. Over the course of 21 months, 495 patients had received at least one dose of pembrolizumab and were included in an analysis of AE and overall efficacy. Three dosing schedules were evaluated: 2 mg per kg every 3 weeks, 10 mg per kg every 3 weeks, and 10 mg per kg every 2 weeks. Tumors were also tested for PD-L1 expression using an anti-PD-L1 antibody clone 22C3 (Merck) with a prototype assay developed by Dako. Expression was reported as the percentage of tumor cells with staining for membranous PD-L1. Strong expression was noted to be >50% positive, weak expression determined to be 1–49% positive, and negative expression <1% positive.
At the time of submission for publication patients had been followed for a median of 10.9 months. Among the all treated patients, the response rate (RR) was 19.4% with best overall response as stable disease in 21.8% of patients. It is noteworthy that response was independent of dosing schedule or histologic type of NSCLC, but current or former smokers had twice the RR of never-smokers. The median duration of response was 12.5 months, with treatment naïve patients achieving a median duration of response of 23.3 months; nearly twice the duration of response of previously treated patients. The median overall survival (mOS) was 12.0 months; median progression-free survival (mPFS) was 3.7 mos.
In patients with strong PD-L1 expression (>50% positive), the RR was 45.2%. When all patients with strong PD-L1 expression were included, the mPFS was 6.3 months, with treatment naïve patients having a mPFS of 12.5 months. The mOS was not reached in treatment naïve patients. PFS and OS were shorter in patients with 1–49% and <1% PD-L1 expression, but interestingly, the RR was 8.3% in patients with negative PD-L1 expression with mPFS of 3.5 months and mOS of 10.4 months. The authors concluded that observations suggest tumor responses have a positive correlation with degree of PD-L1 expression based on their assay, but the small numbers in this evaluation limited the ability to determine a threshold that would exclude non-responders.
Treatment-related adverse events (AE) were reported in 70.9% of patients with no significant difference detected in the three dosing schedules. Grade 3 or higher AE were reported in less than 10% of patients. The most common AE of any grade reported were fatigue (19.4%), pruritus (10.7%), and decreased appetite (10.5%). Immune-related events included hypothyroidism (6.9%) and pneumonitis (3.6%) with grade 3 or greater pneumonitis diagnosed in 9 patients and accounting for 1 death (15).
These data lead to the Phase II/III KEYNOTE-010 randomized, open-label multinational trial comparing docetaxel (75 mg/meter squared every three weeks to pembrolizumab at either 2 or 10 mg/kg in metastatic non-small cell lung cancer with a measured PD-L1 expression of 1% or greater after progression on a platinum doublet or tyrosine kinase inhibitor for those tumors with actionable mutations. Between August 2013 and February 2015, 1,034 patients were enrolled and randomized in a 1:1:1 fashion. They were followed for a median of 13.1 months, and no crossover was allowed. Primary endpoints were OS and PFS in all three groups with comparison of weak and strong PD-L1 expression. Secondary endpoints were safety, RR, and duration of response.
In patients with strong (>50%) PD-L1 expression, OS and PFS were significantly prolonged for both pembrolizumab doses compared with docetaxel. The pembrolizumab 2 mg/kg group, had a RR of 30% and mOS of 14.9 months; and pembrolizumab 10 mg/kg had a RR of 29% with mOS of 17.3 months. This is compared to a RR of 8% and mOS of 8.2 months in the docetaxel group. Comparing the pembrolizumab groups with docetaxel group, the hazard ratio for OS was 0.54 in the pembrolizumab 2 mg/kg group and 0.5 in the 10 mg/kg group versus docetaxel. Median PFS was 5.0 months, 5.2 months, and 4.1 months in pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg, and docetaxel groups, respectively. PFS of both pembrolizumab dose levels compared with docetaxel reached statistical significance (HR =0.59 in 2 mg/kg and 0.59 in 10 mg/kg groups). At any level of positive expression of PD-L1, there was still an OS advantage at both dose levels of pembrolizumab compared to patients treated with docetaxel. The RR was 18% and mOS was 10.4 months in the pembrolizumab 2 mg/kg group; RR was 18% with mOS of 12.7 months in the pembrolizumab 10 mg/kg group, and RR 9% with mOS 8.5 months in docetaxel group. The HR for median OS with pembrolizumab 2 mg/kg versus docetaxel was 0.71 and 0.61 with pembrolizumab 10mg/kg vs. docetaxel.
The one year OS was similar in the two pembrolizumab groups (43.2% for 2 mg/kg and 52.3% in 10 mg/kg) for strong PD-L1 expression (HR =1.12) as well as weak PD-L1 expression (HR =1.17). This is in contrast to docetaxel one year OS of 34.6%.
Interestingly, for the total population, no statistical difference in PFS between either pembrolizumab dose level and docetaxel for PD-L1 expression was identified. Median PFS was 3.9 months, 4.0 months, and 4.0 months in the pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg, and docetaxel groups, respectively with HR of 0.88 and 0.79 in pembrolizumab 2 mg/kg versus docetaxel and pembrolizumab 10 mg/kg versus docetaxel. This effect did not differ by nonsquamous vs. squamous histology (16). These data are presented in Table 2.
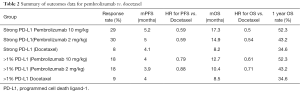
Full table
The spectrum of toxicities also differed for pembrolizumab versus docetaxel treatment arms. Any grade AE occurred in 66% of both pembrolizumab dose levels compared to 81% of the docetaxel group. Grade 3 or higher events were also higher in the docetaxel group (35%) compared to pembrolizumab 2 mg/kg (13%) and 10 mg/kg (16%). AE unique to pembrolizumab included hypothyroidism (8% at both dose levels), hyperthyroidism (4% and 6% at the lower and higher doses respectively), and pneumonitis occurring in 5% and 4% in the respective dose levels. Several other autoimmune complications were identified occurring in 1% or less of the treated patients, namely severe skin reactions, colitis, pancreatitis, adrenal insufficiency, myositis, thyroiditis, hepatitis, hypophysitis, and type 1 diabetes.
The benefit of pembrolizumab over docetaxel in terms of prolonged OS and decreased toxicity led to the approval of pembrolizumab 2 mg/kg every 3 weeks as a viable second line option in advanced NSCLC with PD-L1 expression 1% or greater. These data indicated that, again, strong PD-L1 expression was associated with an even greater RR, OS, PFS, and 1-year survival rate compared with weak PD-L1 expression.
Nivolumab
Bristol-Myers Squibb elected to perform sister studies that separated squamous and non-squamous histology of NSCLS patients. Similar to the Keynote-010, CheckMate 017 compared Nivolumab 3 mg/kg every 2 weeks with docetaxel 75 mg/meter squared every 3 weeks in the randomized phase III international trial in stage IIIB or IV squamous NSCLC after prior treatment with one platinum-containing regimen. Over 15 months, 272 patients were enrolled and treated with either nivolumab or docetaxel. They were followed for a minimum of 11 months. The primary end-point was OS. Secondary endpoints included objective response, PFS, patient-reported outcomes, efficacy according to PD-L1 expression, and safety.
Nivolumab demonstrated a clear advantage over docetaxel in patients with squamous histology. The mOS for nivolumab was 9.2 months compared with 6.0 months for docetaxel (HR =0.59) One-year OS was 42% for nivolumab and 24% for docetaxel. Objective responses were confirmed in 20% of nivolumab-treated patients compared with 9% of docetaxel patients (P=0.008). Time to response was comparable in both groups at 2.2 and 2.1 months, respectively. Median duration of response was not reached in the nivolumab group compared with 8.4 months in the docetaxel groups. Median PFS was 3.5 months in the Nivolumab group and 2.8 months in the docetaxel group with HR of 0.62. The rate of 1-year PFS was also significantly better with nivolumab compared with docetaxel, 21% and 6%, respectively. This trend was observed in all groups except those with two prior therapies, patients over age 75 and patients in Central and South-American countries.
While PD-L1 expression was not a requirement for entry into the study, 88% of patients who were treated had quantifiable expression using a PD-L1 antibody 28-8 created by Epitomics and an IHC assay developed by Dako. Positive PD-L1 expression was balanced among the treatment groups. They did not find a statistically significant difference in terms of RR, OS, or PFS among their prespecified expression levels of <1%, 1%, 5%, and 10%. Interestingly, the investigators observed that PD-L1 negative tumors had similar rates of objective response compared to PD-L1 positive tumors, and rates of objective response were higher in the nivolumab group than the docetaxel group regardless of PD-L1 expression. They concluded that tumor expression of PD-L1 was not necessary to receive a benefit from Nivolumab; however, the method of testing, cut-off parameters, and age of tested tissue were co-founding variables.
Similarly to the pembrolizumab data, the safety profile favored nivolumab over docetaxel. AE of any grade was 58% in the nivolumab group with 7% having a grade 3 or 4 event compared with 86% any grade and 55% grade 3 or 4 in the docetaxel group. Pneumonitis occurred in 5% of the nivolumab patients with no treatment-related deaths reported. Similar rates of rash, pyrexia, and arthralgia were observed (17). These data ultimately led to the FDA approval of Nivolumab 3 mg/kg every 2 weeks in advanced squamous NCSLC after prior platinum therapy.
CheckMate 057 compared Nivolumab 3 mg/kg every 2 weeks with docetaxel 75 mg/square meter every 3 weeks in non-squamous NSCLC in stage IIIB or IV patients previously treated with a platinum-containing regimen. This international phase III trial enrolled and treated 555 patients randomized to nivolumab or docetaxel therapy and followed them for a minimum of 13.2 months. The primary endpoint was OS. Secondary endpoints included rate of investigator-assessed confirmed OR, PFS, patient-reported outcomes, efficacy according to PD-L1 expression, and safety.
Again, Nivolumab demonstrated an overall survival benefit with mOS of 12.2 months compared with 9.4 months in the docetaxel group (HR =0.73). One year OS rate was 51% with nivolumab versus 39% in the docetaxel group. Objective response was higher with Nivolumab than docetaxel (19% vs. 12%) and median duration of response was 17.2 months in the nivolumab group compared to 5.6 months with docetaxel therapy. The PFS rate at one year was 19% with nivolumab and 8% with docetaxel. The HR for disease progression or death was 0.92, but the authors concluded that numerically, nivolumab is still favored in terms of PFS for all subgroups except those on third-line therapy, those in non-US and Europe regions, never smokers, EGFR mutation positive, and KRAS wild type.
As with the sister study, Checkmate 017, a large percentage of patients had tissue that was evaluable for PD-L1 expression (78%), and a post hoc analysis indicated that there was a strong predictive association between PD-L1 expression and clinical outcomes. The authors note that no meaningful overall survival advantage was noted with nivolumab over docetaxel in the non-PD-L1 expression tumors, but that at any level of expression 1% or greater, there was a meaningful separation of the survival curves. As would be predicted based on the pembrolizumab data, the magnitude of benefit was greater with increasing PD-L1 expression for all indices.
The safety profile of nivolumab was superior to that of docetaxel in terms of any grade toxicity and grade 3 or 4 toxicity for non-squamous histology; however, discontinuation of study drug due to treatment-related AE occurred in 5% of nivolumab patients, mostly due to pneumonitis occurring in 1% of patients treated with nivolumab. There was one death in the nivolumab group due to encephalitis (18).
Atezolizumab
POPLAR was an international phase II randomized clinical trial comparing Atezolizumab with docetaxel in advanced or metastatic NSCLC as second and third-line therapy after progression on platinum chemotherapy. Over 18 months, 177 patients were enrolled and randomized to receive either atezolizumab at 1,200 mg or docetaxel 75 mg/meter squared every 3 weeks. Patients were followed for a median of 14.8 months in the atezolizumab group and 15.7 months in the docetaxel group. The primary endpoint was OS in the overall population as well as within the PD-L1 subgroups. Secondary end-points included ORR, PFS, duration of response, and efficacy according to immune-modified RECIST designed to further describe the unconventional responses sometimes observed with immunotherapy (19), atezolizumab pharmacokinetics, patient-reported outcomes, and biomarkers, and pharmacodynamics. Unique to this study was the evaluation of not only PD-L1 expression of the tumor cells (TC3 ≥50%; TC2: 5–50%; TC1: 1–5%; and TC0 <1%) but also PD-L1 expressing tumor-infiltrating immune cells status as a percentage of the tumor area (IC3 ≥10%; IC2: 5–10%; IC1: 1–5%; and IC0 <1%) using the anti-PD-L1 clone SP142 in the IHC assay by Roche® (20).
As would be expected based on prior immunotherapy trials in this second or more-line treatment cohort, atezolizumab was superior to docetaxel. Atezolizumab demonstrated a clear benefit over docetaxel in the entire intent-to-treat population with a larger benefit seen in those patients whose tumors had increased levels of PD-L1 expression. In addition, the expression of PD-L1 on tumor-infiltrating immune cells was also associated with an increased benefit. For the entire population, mOS was 12.6 vs. 9.7 months with docetaxel (HR =0.73). Overall survival in squamous histology favored atezolizumab over docetaxel, (HR =0.8) and those with non-squamous histology had a more pronounced OS benefit (HR =0.69). Compared to patients on docetaxel with corresponding PD-L1 expression on tumor and tumor-infiltrating cells, the OS benefit of atezolizumab was more pronounced in the TC 2/3 or IC 2/3 (HR =0.54) and in the TC 1/2/3 or IC 1/2/3 groups (HR =0.59). OS in TC0 or IC0 were similar for docetaxel and atezolizumab.
PFS was not significantly longer nor RR higher in the atezolizumab group compared to docetaxel, but when analyzed for PD-L1 status, there was an observed trend favoring atezolizumab over docetaxel for positive PD-L1 expression. The only groups with a statistically significant improvement in RR or PFS with atezolizumab were the TC3 and IC3 subgroups. Duration of response was still longer for atezolizumab (median of 14.3 months) compared with docetaxel (median of 7.2 months). PD-L1 expression on either the tumor cells or the tumor-infiltrating immune cells was associated with benefit. Only 1% of patients had TC3 and IC3. When analyzing the non-overlapping subgroups, they found that a significant prolongation in OS was still observed if a tumor had measurable PD-L1 expression on either tumor cell or tumor-infiltrating cell. Unfortunately, the moderate study size limits the ability to draw conclusions in the small sub-group analysis. The PD-L1 expression of either cell was not correlated with the efficacy of docetaxel, suggesting that this biomarker is predictive of response to this immunotherapy, not just a prognostic biomarker of a more favorable tumor biology.
Atezolizumab also appears to be associated with less toxicity compared to docetaxel. In the atezolizumab group, 40% of patients experienced grade 3–4 AE compared with 53% in the docetaxel group. The most common grade 3–4 AE in the atezolizumab group were pneumonia and increased aspartate aminotransferase, both of which occurred in 2% of the treated population. Treatment discontinuation occurred in 8% of the atezolizumab group compared with 22% in the docetaxel group (21). With this data, the OAK phase 3 study of atezolizumab versus docetaxel in the front line setting was initiated (22).
The OAK phase III multinational study compared atezolizumab versus docetaxel after progression on platinum-based therapy and enrolled 1,225 patients with any PD-L1 status. The primary endpoint was OS in the total population as well as in patients with PD-L1 expression on >1% or tumor cells or infiltrating immune cells. Secondary endpoints were objective RR, PFS, duration of response, and safety. The outcomes were presented at the European Society for Medical Oncology 2016 congress.
The mOS, 12 month OS rate, and 18 month OS rate for the atezolizumab group compared with the docetaxel group was 13.8 vs. 9.6 months (HR =0.73), 55% vs. 41%, and 40% vs. 27%, respectively. This OS advantage with atezolizumab did not differ significantly by tumor histology (squamous vs. non-squamous). No difference in PFS was noted. When evaluating the relationship between outcomes and PD-L1 expression, a notable trend in favor of atezolizumab treatment was observed. In TC0 or IC0 patients (45% of patients), the mOS was significantly longer with atezolizumab therapy (12.6 months) than with docetaxel therapy (8.9 months, HR =0.75). In patients with any degree of PD-L1 expression ≥1% (TC1/2/3 or IC1/2/3, 55% of patients), the survival curves offered greater separation with mOS of 15.7 months with atezolizumab and 10.3 months with docetaxel (HR =0.74). In the group with PD-L1 expression ≥exp% (TC3 or IC3, 16% of patients) the greatest difference was observed, with mOS of 20.5 months for atezolizumab and 8.9 months with docetaxel (HR =0.41).
Safety evaluations favored atezolizumab as well. Treatment related AE, treatment-related grade 3–5 AE, and AE leading to study treatment withdrawal for atezolizumab vs. docetaxel were 64% vs. 86%, 15% vs. 43.2%, and 8% vs. 19%, respectively. The rate of immune-related AE was minimal with atezolizumab-treated patients experiencing pneumonitis, hepatitis, and colitis at rates of 1.7%, 0.6%, 0.3%, respectively. The authors concluded that atezolizumab is beneficial, safe, and tolerable without any new safety signals as the second or further line treatment (23).
Advanced NCSLC, front line therapy
Nivolumab
CheckMate 012 is a phase I study assessing safety and efficacy of single agent nivolumab, nivolumab with erlotinib, nivolumab with bevacizumab, nivolumab with platinum-based doublet chemotherapy, or combination immunotherapy of nivolumab with ipilimumab in Stage IIIB or IV any-histology NSCLC patients who had not received prior therapy (24). Data thus far published or presented is below.
Single-agent nivolumab 3 mg/kg given every 2 weeks until progression or unacceptable toxicity was evaluated in 52 patients. The primary end-point was safety with secondary endpoints including ORR, PFS, and OS. The rate of AE of any grade was comparable to prior studies at 71% with only 12% of patients discontinuing the study due to an adverse event. Fatigue, rash, nausea, diarrhea, pruritus, and arthralgia all occurred in less than 30% of patients. Grade 3–4 AE were reported in 19% of patients, with rash being the most common. The ORR was 23%, and was slightly higher in patients with PD-L1 expression (28%). Median OS was 19.4 months. The one-year OS rate was 73% and 18 month OS rate was 57%. Median PFS was 3.6 months with 24-week PFS rate of 41% (25). This led to the phase II/III CheckMate 026 trial. Unfortunately, a BSM shareholder report indicates that this trial did not meet its primary end point of PFS compared to chemotherapy in treatment naïve patients with at least 5% PD-L1 expression (26). The final data publication is still pending at time of this publication.
Nivolumab in combination with platinum-based doublet chemotherapy was evaluated in 56 patients in 4 treatment groups: nivolumab 10 mg/kg plus gemcitabine and cisplatin every 3 weeks (squamous histology), nivolumab 10 mg/kg plus pemetrexed and cisplatin every 3 weeks (non-squamous histology), nivolumab 10 mg/kg plus paclitaxel and carboplatin every 3 weeks (all histology), or nivolumab 5 mg/kg plus paclitaxel and carboplatin every 3 weeks (all histology). No dose limiting toxicities were reported during the first 6 weeks of therapy. The grade 3 or 4 AE rate was 45%. The discontinuation rate due to AE was 21%, and this is noted to be nearly twice that of the single-agent arm. The objective RR ranged from 33% in the nivolumab plus gemcitabine and cisplatin group to 47% in the nivolumab plus pemetrexed and cisplatin and the nivolumab 10 mg/kg plus paclitaxel and carboplatin groups. The 24-week PFS rate was 38% in the nivolumab 10 mg/kg plus paclitaxel and carboplatin, 71% in the nivolumab plus pemetrexed and cisplatin group, and 51% in the other 2 groups. Two-year OS rates ranged from 25% in the nivolumab plus gemcitabine with cisplatin to 62% in the pembrolizumab 5 mg/kg plus paclitaxel and carboplatin group (27).
An abstract of combination ipilimumab and nivolumab in advanced NSCLC as first line treatment has been presented. One hundred-forty-eight patients were randomized to nivolumab with ipilimumab every 3 weeks for 4 weeks followed by nivolumab every 2 weeks until progression or toxicity (Schema 1), nivolumab every 2 weeks with ipilimumab every 6 weeks (Schema 2), higher dose nivolumab every 2 weeks and ipilimumab every 12 weeks (Schema 3), and higher dose nivolumab every 2 weeks with ipilimumab every 6 weeks (Schema 4). Treatment-related AE rates ranged 69% in schema 4% to 77% in schema 1. The discontinuation rate was 10%, which was comparable to the discontinuation rate of Nivolumab as a single agent, and much lower than the nivolumab plus platinum-based chemotherapy doublet arm. No deaths were attributed to the treatment. The most commonly reported grade 3–4 AE were dermatologic (3–15%), gastrointestinal (0% to 8%) endocrine (3% to 8%), and hepatic 5% to 10%). Overall RR ranged from 13% in schema 1to 39% in schema 3. The mOS was not yet reached in any group, and mPFS ranged from 4.9 in schema 2 to 10.6 months in schema 1. The 24-week PFS rate was not yet calculated in schemas 2 and 4, but reached 55% in schema 1 and 63% in schema 3 (28).
Pembrolizumab
KEYNOTE 021 was the phase II multicohort international trial. One cohort compared pembrolizumab 200 mg plus carboplatin area under the curve of 5 mg/mL per min and pemetrexed 500 mg/meter squared every 3 weeks followed by pembrolizumab for 24 months with pemetrexed maintenance therapy with carboplatin and pemetrexed alone followed by pemetrexed maintenance therapy in stage IIIB or IV non-squamous NSCLC without targetable EGFR, ALK, or ROS1 genetic aberrations stratified by PD-L1 proportion score (<1% vs. ≥1%). The primary endpoint was objective RR with secondary endpoints of PFS duration of response, OS, and correlation between PD-L1 expression and outcomes in the intention-to-treat population. Over 25 months, 123 patients were enrolled in a 1:1 fashion. They were followed for a median of 10.6 months and 32% of patients in the chemotherapy group crossed-over pembrolizumab monotherapy at first progression.
In the chemotherapy alone group, objective RR was 29% compared with 55% in the pembrolizumab plus chemotherapy group (P=0.0016). Median time to response was 2.7 months in the chemotherapy group and 1.5 months in the pembrolizumab plus chemotherapy group. The mPFS for chemotherapy was 8.9 months, which was significantly shorter than the mPFS of 13.0 months for pembrolizumab plus chemotherapy (HR of 0.53). No difference in OS was noted, however.
The safety profile was similar between the 2 treatments with 90% of the chemotherapy only and 93% of the pembrolizumab plus chemotherapy patients experiencing AE of any degree. Thirteen percent of the chemotherapy group and 10% of the pembrolizumab plus chemotherapy group discontinued the study treatment due to treatment-related AE. There were 3 deaths attributed to study treatment: 2 in the chemotherapy group (sepsis and pancytopenia) and 1 in the pembrolizumab group (sepsis). Grade 3 or higher toxicities occurred in 26% of the chemotherapy group compared with 39% in the pembrolizumab group.
When evaluating the relationship between PD-L1 expression and outcomes, the authors note that there was no difference in objective response in patients with <1% or ≥1%, but there was a trend demonstrating increased objective RR with >50% PD-L1 expression. The small sample size limited the ability to make statistical comparisons. The phase III KEYNOTE 189 and KEYNOTE 407 trial will likely answer that question in non-squamous and squamous NSCLC, respectively (29).
KEYNOTE 024 was a phase III open-label trial comparing single agent pembrolizumab every 3 weeks with platinum-based doublet of the physician’s choice (gemcitabine or pemetrexed with cisplatin or carboplatin or carboplatin with paclitaxel) in the first line setting for advanced or metastatic NCSLC. The trial design limited participation to those patients with tumors that had PD-L1 expression of at least 50% using the pembrolizumab companion IHC testing at a central lab, and did not express EGFR mutations or ALK rearrangements. From September 2014 to October 2015, 305 patients were enrolled and assigned in a 1:1 manner to chemotherapy platinum doublet followed by maintenance if indicated vs. Pembrolizumab at 200 mg IV every 3 weeks for 35 cycles.
In the intention to treat population, the mPFS was 10.3 months in the pembrolizumab group and 6 months in the chemotherapy group (P<0.001) with benefit of pembrolizumab evident in all treatment groups. It is worth noting that the HR for never smokers was 0.9, but still favored pembrolizumab. The 6 month survival rate was 80.2% in the pembrolizumab group compared with 72.4% in the chemotherapy group. The mOS was not reached in either group at the second interim analysis; however, pembrolizumab group had longer OS than the chemotherapy group with HR for death 0.6. Higher objective RR was observed in the pembrolizumab group (69% vs. 42%) with time to response of 2.2 months in both groups, but median duration of response not reached in the pembrolizumab group versus the chemotherapy group response duration of 6.3 months.
Safety analysis demonstrated fewer grade 3, 4, or 5 AE with pembrolizumab than with chemotherapy, similar rates of discontinuation due to AE, and fewer deaths related to AE. Unique to pembrolizumab were the higher rates of pneumonitis, skin reactions, colitis, myositis, hypophysitis, nephritis, pancreatitis, and type 1 diabetes compared with chemotherapy (30,31). Overall pembrolizumab was much better tolerated and with improved RR, OS, PFS, and duration of response, pembrolizumab received front-line approval from the FDA.
Small cell lung cancer (SCLC)
Ipilimumab
A phase III, double-blinded placebo-controlled study of combination checkpoint inhibitor plus chemotherapy in newly diagnosed advanced stage SCLC comparing etoposide and platinum +/– ipilimumab 10 mg/kg every 3 weeks for a total of 4 doses followed by ipilimumab or placebo maintenance every 12 weeks enrolled and treated 954 patients. Primary end point was OS.
No difference in OS was observed between the 2 treatment groups, with combination checkpoint inhibitor and chemotherapy achieving a mOS of 11 months and chemotherapy plus placebo achieving mOS of 10.9 months (HR =0.94). PFS was also no different between the two treatments. The mPFS for the combination was 4.6 and 4.4 months for chemotherapy plus placebo (HR=0.85). Eighteen percent of patients discontinued combination therapy compared with 2% on chemotherapy plus placebo due to treatment related AE. There were 5 reported treatment-related deaths with combination therapy compared with 2 deaths in the chemotherapy plus placebo group (32).
Nivolumab and ipilimumab
CheckMate 032 was a phase I/II international open-label study evaluating safety and efficacy of single agent nivolumab and combination ipilimumab and nivolumab in limited or extensive stage small cell lung cancer after progression on one or more previous chemotherapy regimens including a platinum-based regimen. From November 2013 to July 2015, 216 patients were sequentially enrolled and treated on one of the following schedules: nivolumab 3 mg/kg every 2 weeks, or nivolumab 1 mg/kg with ipilimumab 1 mg/kg every 3 weeks (dose level 1), or nivolumab 1 mg/kg with ipilimumab 3 mg/kg every 3 weeks (dose level 2), or nivolumab 3 mg/kg with ipilimumab 1 mg/kg every 3 weeks (dose level 2b). Each of the combinations regimens was given for 4 cycles followed by nivolumab 3 mg/kg every 2 weeks until toxicity or progression. Patients with progression on single agent nivolumab were allowed to crossover to the combination arms. The primary endpoint was objective response. Secondary endpoints were OS, PFS, duration of response, and safety. The relation of PD-L1 expression status with efficacy was evaluated retrospectively.
After a minimum follow-up of 12 weeks, analysis was undertaken. The cohorts were too small to detect statistical significance among the treatment groups, but responses and survival data were as follows. The objective RR was 10% for single-agent nivolumab, 23% for dose level 2, and 19% for dose level 2b. One complete response was observed at dose level 2. Partial response was observed in 10% of single agent nivolumab, 21% of dose level 2, and 19% of dose level 2b. Stable disease was achieved in 22% of single-agent nivolumab, 21% of dose level 2, and 17% of dose level 2b. The median duration of response was not reached for single agent nivolumab, 7.7 months for dose level 2, and 4.4 months for dose level 2b. Median OS was 4.4 months for single-agent nivolumab, 7.7 months at dose level 2, and 6.0 months at dose level 2b. One year OS rate was 33% for nivolumab alone, 43% for dose level 2, and 35% for dose level 2b. The median PFS was 1.4 months for single-agent nivolumab, 2.6 months for dose level 2, and 1.4 months for dose level 2b. The 1-year PFS rate was 11% for nivolumab alone, 19% for dose level 2, and the 1 year milestone was not yet reached for dose level 2b at time of publication of the data. The single-agent nivolumab and dose level 2 combinations appeared to have flattening of the survival curves suggesting an ongoing benefit. As with prior immunotherapy studies, the impact on OS was more pronounced than on PFS, suggestive again, of the unique treatment benefit with checkpoint inhibitors that is sometimes observed.
PD-L1 expression was evaluable in 69% of the patients (fresh or archived specimens). Only 17% of patients had at least 1% expression and 5% had 5% or greater expression. While the data was not powered to detect a statistical difference, tumor responses were observed in patients regardless of their PD-L1 expression level.
A safety analysis of nivolumab alone or in combination with ipilimumab in SCLC indicated overall tolerability. Only 6% of patients in the nivolumab group discontinued due to toxicity. The dose level 2b group had 7% discontinuation due to toxicity and 1 treatment-related death from pneumonitis. The dose level 2 group, with the higher ipilimumab dose, had an 11% discontinuation rate due to toxicity and 2 treatment-related deaths, one each due to myasthenia gravis and progressive renal failure. The total percentage of grade 1–2 AE with single agent nivolumab was 40%, 9% grade 3, and 4% grade 4. Grade 1–2 AE occurred in 49% of patients receiving dose level 2, 23% grade 3, and 7% grade 4. Dose level 2b patients experienced grade 1–2 AE at a rate of 56%, 15% grade 3, and 4% grade 4. Events that occurred at a rate of greater than 10% in any treatment group were fatigue, pruritus, diarrhea, decreased appetite, hyperthyroidism, hypothyroidism, and rash.
Based on these results, phase III studies have been initiated (33).
Pembrolizumab
KEYNOTE-028 is an ongoing trial assessing Pembrolizumab 10 mg/kg in SCLC after progression on a platinum-based regimen in patients with at least 1% PD-L1 expression in the tumor cells or stroma. Data on the first 16 patients has been presented. Nine participants experienced an AE and only one had a grade 3 or greater adverse event. There were no discontinuations due to AE suggesting that it is safe. Four patients have had a partial response and one has stable disease (34)
Advanced, chemotherapy-resistant mesothelioma
Tremelimumab was evaluated in a phase II trial MESOTTREM-2008 which enrolled and treated 29 patients who had inoperable mesothelioma with measureable disease after front line treatment with platinum-based chemotherapy. It was dosed at 15 mg/kg IV every 90 days until progression or treatment-limiting toxicity. The primary endpoint was objective RR with additional endpoints of disease control rates, PFS, OS, and safety.
After median follow-up of 27 months, there were no complete responses, 2 partial responses lasting 6 and 18 months, 7 patients with stable disease of a median duration of 12.4 months, and 20 patients with progressive disease. The mOS was 10.7 months and mPFS of 6.2 months. There was a noted non-significant association between epithelioid histology and clinical benefit or survival.
The safety analysis showed 13% of patients experienced grade 3 or 4 AE including colitis, elevations in liver enzymes, elevations in pancreatic enzymes, and peripheral neuropathy. No patients died from the treatment, and all AE were treated with steroids and/or intravenous immunoglobulin. Phase III trials are now underway (14).
Predicting response to checkpoint inhibitor therapy
This novel treatment approach is being tested in nearly every tumor type with mixed outcomes. Efforts at predicting responders beyond PD-L1 expression are ongoing. Growing evidence supports the idea that tumors with higher mutational burdens tend to have increased benefit from checkpoint inhibitor therapies, as seen in melanoma and lung cancer, where this type of therapy gained momentum (35,36). One known factor leading to the high mutagenesis in lung cancer is tobacco smoke (37). In lung cancer specifically, there was a strongly positive association between a high frequency of mutations and improved RR, PFS, and durable clinical benefit with pembrolizumab, suggesting there may even be a threshold effect. The molecular signature of smoking-related mutations was identified, and the higher frequency of this signature strongly correlated with a more robust the clinical benefit from pembrolizumab compared with patients whose tumors harbored non-smoking-related mutations (38).
Another interesting observation with growing attention has been the Abscopal effect, where local treatment of a single metastatic lesion leads to regression of non-treated lesions. For example, it was observed that radiation to a NSCLC lesion caused a temporary increase in a biomarker of radiation-related DNA-damage in circulating lymphocytes while undergoing radiation; however there was a delayed increase in the same biomarker in eyebrow hairs which were not in the radiation field, suggesting that local radiation induces systemic DNA damage (39). Now, several studies are using this and other similar observations to logically combine radiation and checkpoint inhibitor therapy (40).
Conclusions
At this time, single agent nivolumab, pembrolizumab, and atezolizumab are approved for second-line treatment after platinum-based chemotherapy in advanced NSCLC. As front-line therapy in patients with metastatic disease, nivolumab did not demonstrate efficacy in an unselected population, while pembrolizumab demonstrated improved outcomes by limiting trial participants to those with strong PD-L1 expression. In SCLC, combination nivolumab and ipilimumab has shown promise as second line therapy after platinum-based therapy regardless of PD-L1 expression. Tremelimumab has some efficacy in mesothelioma, but phase III trials are ongoing.
Several additional checkpoint inhibitors are under development and being used in clinical trials with the hope that this novel approach to cancer treatment, perhaps alone or in combination with chemotherapy or other targeted agents will continue to revolutionize cancer care.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Marin Feldman Xavier) for the series “Advances on Clinical Immunotherapy” published in Translational Cancer Research. The article has undergone external
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.02.07). The series “Advances on Clinical Immunotherapy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012, version 1.0. Cancer incidence and mortality worldwide: IARC Cancer Base No. 11. Lyon, France: International Agency for Research on Cancer 2013. Available online: http:globocan.iarc.fr
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [Crossref] [PubMed]
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. [Crossref] [PubMed]
- Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365-9. [Crossref] [PubMed]
- Schneider H, Downey J, Smith A, et al. Reversal of the TCR stop signal by CTLA-4. Science 2006;313:1972-5. [Crossref] [PubMed]
- Riley JL, Mao M, Kobayashi S, et al. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci U S A 2002;99:11790-5. [Crossref] [PubMed]
- FDA approves Keytruda for advanced non-small cell lung cancer. fda.gov. U.S. Food and Drug Administration. 5 Oct. 2015. Web. 8 Oct 2016. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm465444.htm
- Nivolumab Injection. FDA.gov. U.S. Food and Drug Administration, 13 Oct. 2015. Web. 8 Oct. 2016. Available online: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm526430.htm
- Atezolizumab. FDA. gov. U.S. Food and Drug Administration, 18 October 2016. Web. 16 Feb. 2017. Available online: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm466576.htm
Atezolizumab (TECENTRIQ) - FDA approves new treatment for a type of late-stage skin cancer. fda.gov. U.S. Food and Drug Administration. 25 Mar. 2011. Web 16 Feb. 2017. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm1193237.htm
- Ipilimumab. 9 Oct. 2016. Available online: https://www.clinicaltrials.gov/
- Calabrò L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol 2013;14:1104-11. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Boyd Z, Smith D, Baker B, et al. Development of a PD-L1 companion diagnostic IHC assay (SP142) for atezolizumab. [abstract]. In: Proceedings of the CRI-CIMT-EATI-AACR Inaugural International Cancer Immunotherapy Conference: Translating Science into Survival; September 16-19, 2015; New York, NY. Philadelphia (PA):AACR; Cancer Immunol Res 2016;4(1 Suppl):Abstract nr B001.
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- A Randomized Phase 3 Study of Atezolizumab (an Engineered Anti-PDL1 Antibody) Compared to Docetaxel in Patients With Locally Advanced or Metastatic Non-Small Cell Lung Cancer Who Have Failed Platinum Therapy - "OAK". U.S. National Institutes of Health. 2013.
- Barlesi F, Park K, Ciardiello F, et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in advanced NCSLC. Paper presented at: ESMO Congress 2016. Proceedings of the 41st European Society for Medical Oncology Congress on Disease Treatment to Patient Care; 2016 Oct 7-11, Copenhagen, Denmark.
- Study of Nivolumab (BMS-936558) in Combination With Gemcitabine/Cisplatin, Pemetrexed/Cisplatin, Carboplatin/Paclitaxel, Bevacizumab Maintenance, Erlotinib, Ipilimumab or as Monotherapy in Subjects With Stage IIIB/IV Non-small Cell Lung Cancer (NSCLC) (CheckMate 012). U.S. National Institutes of Health. 2011.
- Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2980-7. [Crossref] [PubMed]
- Nivolumab did not meet primary endpoint of progression-free survival in NSCLC in CheckMate-026 trial. Available online: http://www.ascopost.com/
- Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:2969-79. [Crossref] [PubMed]
- Hellmann MD, Gettinger SN, Goldman JW, et al. CheckMate 012: Safety and efficacy of first-line (1L) nivolumab (nivo; N) and ipilimumab (ipi; I) in advanced (adv) NSCLC. J Clin Oncol 2016;34:abstr 3001.
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Brahmer JR, Kim ES, Zhang J, et al. KEYNOTE-024: Phase III trial of pembrolizumab (MK-3475) vs platinum-based chemotherapy as first-line therapy for patients with metastatic non-small cell lung cancer (NSCLC) that expresses programmed cell death ligand 1 (PD-L1). J Clin Oncol 2015;33:abstr TPS8103.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Reck M, Luft A, Szczesna A, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Ott PA, Fernandez ME, Hiret S, et al. Pembrolizumab (MK-3475) in patients (pts) with extensive-stage small cell lung cancer (SCLC): Preliminary safety and efficacy results from KEYNOTE-028. J Clin Oncol 2015;33:abstr 7502.
- Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214-8. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Pfeifer GP, Denissenko MF, Olivier M, et al. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 2002;21:7435-51. Review. [Crossref] [PubMed]
- Hellmann M, Rizvi N, Wolchok JD, et al. Genomic profile, smoking, and response to anti-PD-1 therapy in non-small cell lung carcinoma. Mol Cell Oncol 2015;3:e1048929 [Crossref] [PubMed]
- Siva S, Lobachevsky P, MacManus MP, et al. Radiotherapy for Non-Small Cell Lung Cancer Induces DNA Damage Response in Both Irradiated and Out-of-field Normal Tissues. Clin Cancer Res 2016;22:4817-26. [Crossref] [PubMed]
- Walshaw RC, Honeychurch J, Illidge TM. Stereotactic ablative radiotherapy and immunotherapy combinations: turning the future into systemic therapy? Br J Radiol 2016;89:20160472 [Crossref] [PubMed]



