
Novel immunotherapies and their role in the treatment of acute lymphoblastic leukemia
Introduction
Acute lymphoblastic leukemia (ALL) represents 20% of acute leukemias in adults, and there are about 6,000 new cases diagnosed annually in the United States (1). ALL in the pediatric patient population treated with intensive chemotherapy induction regimens has an overall survival (OS) approaching 90% (2-4). Unfortunately, this is not the case for the majority of adult patients with ALL, and long-term survival is only achieved in roughly 30% of patients (5,6).
Immunotherapy in the form of targeting selective surface antigens on leukemic cells has been a promising adjunct to traditional therapy and is continuously evolving. Therapies against CD20 and CD52 have been previously evaluated and used for quite some time (7,8). However, immunotherapy directed against CD19 and CD22 may be more effective given the more ubiquitous expression in precursor and mature B cells (9). There are many different methods of modulating the immune system and directing immune mediated activity against the cells carrying these antigens. Traditional unconjugated monoclonal antibodies, antibody-drug conjugates, bispecific T cell engagers and chimeric antigen receptor (CAR) T cells are the different modalities of immunotherapy that have been utilized thus far.
Unconjugated monoclonal antibodies
Rituximab
Rituximab is a murine derived monoclonal antibody that targets CD20. Studies investigating the use of rituximab in conjunction with standard induction chemotherapy have been performed by several groups (7,10,11). In one study by Thomas et al., 2 doses of rituximab were added to each of the four cycles of conventional hyper-CVAD to treat patients with Philadelphia chromosome negative (Ph−), CD20+ patients with ALL. Patients who were treated with rituximab in additional to standard induction chemotherapy were found to have an improved 3-year complete remission (CR) rate when compared with historical controls. This was especially evident in younger patients less than 60 years old, where 3-year OS was at 75% in the modified hyper-CVAD plus rituximab group versus 47% in historical controls treated with standard hyper-CVAD (10).
The German multicenter study group evaluated the addition of rituximab to induction chemotherapy in 263 patients with CD20+ ALL. This study demonstrated an improved CR rate and 5-year OS when compared with historical controls (11). CRR at 16 weeks of treatment was 90% vs. 59% in standard risk disease and 64% vs. 40% in high risk disease. The 5-year OS rate was 71% vs. 57% in standard risk disease and 55% vs. 36% in the high risk disease group. It is important to note that both of the above studies are prospective but without randomized controls.
A phase III randomized controlled trial labeled, GRAALL-2005, evaluated standard chemotherapy against standard chemotherapy plus rituximab in 209 patients with newly diagnosed ALL. Both groups had a similar CR rate and level of minimal residual disease (MRD). The rituximab group had lower incidence of relapse at 2 years (18% vs. 30.5%), hence the 2-year event free survival was also improved in the rituximab cohort at 65% vs. 52% in the control group (7).
Ofatumumab
Ofatumumab is a second generation human derived monoclonal antibody against CD20. It binds to a small loop epitope of CD20 and is generally considered more potent than rituximab in terms of greater binding affinity and ability to produce antibody dependent cell mediated cytotoxicity (12,13). A phase 2 study performed by Jabbour et al. investigated the addition of ofatumumab to hyper-CVAD induction in 25 patients with ALL. This study showed a CRR of 96%. The 1-year progression free survival (PFS) was 94% and the OS rate was 92% (14). Additional ongoing studies are examining combination therapy with augmented Berlin-Frankfurt-Munster (BFM) therapy (daunorubicin, vincristine, prednisone, dexamethasone, PEG asparaginase, and methotrexate) and ofatumumab in adolescent and young adult patients aged 12–30 with newly diagnosed ALL (NCT02419469).
Epratuzumab
Epratuzumab is an unconjugated humanized monoclonal antibody that binds to CD22. Upon binding to CD22, the receptor antibody complex becomes internalized. The proposed mechanism of activity includes antibody mediated cell cytotoxicity, CD22 phosphorylation and downstream signaling resulting in B cell modulation and inhibition of cell proliferation. It has been studied in the context of pediatric patients with CD22 positive relapsed or refractory ALL. In the study conducted by the Children’s Clinical Oncology Group, epratuzumab was studied in the relapsed disease setting. It was given initially as single agent therapy twice weekly for 2 weeks, then concurrently with re-induction chemotherapy as 4 weekly doses. There were improved rates of MRD negativity, but there was no difference in remission rates when compared with historical controls (15).
The Southwestern Oncology Group tested epratuzumab in combination with clofarabine and cytarabine in 31 adult patients with relapsed or refractory ALL (SWOG S0910). While there was an increased response rate of 52% compared to 17% in historical controls (SWOG 0530), the median OS remained similar at approximately 5 months (16). There is an international European randomized phase III clinical trial currently ongoing that is comparing epratuzumab plus standard chemotherapy (BFM protocol) with standard chemotherapy alone in first relapse pediatric patients with standard risk ALL (NCT01802814).
Alemtuzumab
Alemtuzumab is a humanized monoclonal antibody against CD52 and causes cell death through antibody dependent cell mediated cytotoxicity. The CALGB 100102 study was a phase I trial that studied alemtuzumab in the setting of post-remission therapy, in between consolidation and maintenance chemotherapy. It was administered 3 times weekly up to a maximum dose of 30 mg. Median OS was 55 months and there was a median 1 log reduction in MRD. However, there were significant adverse effects noted, such as viral infections and myelosuppression (17). A single institution phase II trial studied the use of single agent alemtuzumab in 13 pediatric patients with relapse or refractory ALL, and a CR was only seen in one patient (18). A phase I/II study combined alemtuzumab with intensified combination chemotherapy in upfront treatment for ALL in 302 adult patients and achieved a CRR of around 80% (NCT00061945). Alemtuzumab was also investigated as part of a myeloablative conditioning regimen for stem cell transplantation in 15 patients with high risk ALL, but did not yield promising results (19). While alemtuzumab was safely added to the conditioning regimen, it was proposed that the antileukemic activity of alemtuzumab was nullified by T cell depletion of the donor graft. Because of its modest activity and significant side effects, the path forward for utilizing alemtuzumab in ALL is unclear (20).
Antibody-drug conjugates
Inotuzumab ozogamicin
Inotuzomab ozogamicin is a humanized monoclonal antibody targeting CD22 that is conjugated to calicheamicin. Calicheamicin is a cytotoxic compound that binds to the minor groove of DNA and causes double stranded DNA breaks, eventually resulting in apoptosis. Inotuzumab has been studied for treatment of ALL in the setting of relapsed and refractory disease but also in the upfront setting (21). It has also been used in combination with chemotherapy and as a single agent. An initial MD Anderson study treated 90 adult patients who had relapsed-refractory ALL with single agent inotuzumab. The overall response rate was 58%, with a CR rate of 19%. A large majority of treatment response was also seen after one cycle and a significant majority of patients were able to go on and receive allogeneic stem cell transplant (22). Thrombocytopenia was a common adverse effect as well as neutropenia.
Another MD Anderson clinical trial evaluated the use of inotuzumab and combination chemotherapy in treatment of patients older than 60 with newly diagnosed B cell ALL who were considered to be poor candidates for conventional chemotherapy. The regimen used was augmented hyper-CVAD with overall dose reduction and omission of doxorubicin. Out of 20 patients, CR was 95% compared to 75% in historical controls. An additional study was performed evaluating combination of inotuzumab and the same augmented hyper-CVAD regimen in relapsed or refractory disease, and the ORR was 75% (23). There is an ongoing study by the Southwest Oncology Group (S1312) investigating the use of inotuzumab in combination with CVP for relapsed refractory ALL patients (NCT01925131). In addition, there is also an ongoing trial evaluating use of inotuzumab and bosutinib in CD22+, Ph+, relapsed or refractory ALL (NCT02311998).
Most recently, a phase 3 randomized control clinical trial has been completed comparing the use of single agent inotuzumab against conventional chemotherapy in relapsed or refractory ALL in adult patients. Conventional chemotherapy consisted of one of three regimens: fludarabine and cytarabine, cytarabine and mitoxantrone, or high dose cytarabine alone. There were a total of 326 patients who were randomized into either group. The rate of CR was significantly higher in the inotuzumab group at 80.7% vs. 29.4% in the standard group. The inotuzumab group also had significantly lower negative MRD at 78.4% compared to 28.1% in standard chemotherapy. PFS was also longer in the inotuzumab group at 5 months compared with 1.8 months. The median OS was 7.7 months in the inotuzumab group vs. 6.7 months with standard chemotherapy (24). The most common associated adverse event in the inotuzumab group was veno-occlusive liver disease along with cytopenias.
Combotox
Combotox is a combination antibody-drug conjugate directed against CD19 and CD22. The antibodies are mixed in a 1:1 ratio and are coupled to a deglycosylated ricin A chain. In a phase 1 trial with adult patients with relapsed or refractory ALL, 3 of 17 patients treated with combotox achieved CR. The MTD was determined to be 7 mg/m2 for 3 doses (25). There was a high incidence of severe adverse effects, the primary toxicity being vascular leak syndrome. There is also an ongoing phase 1 trial evaluating the use of conventional chemotherapy with cytarabine plus combotox in the relapsed or refractory adult ALL population (NCT01408160).
CAT 8015—moxetumomab pasudotox
CAT-8015 is a second generation compound that is conjugated with pseudomonas exotoxin and targets CD22. Upon binding to CD22, the compound is internalized and the pseudomonas toxin is eventually activated by the low lysosomal pH, causing ribosomal inactivation and cell death. CAT-8015 has increased stability, binding avidity to CD22 and improved cytotoxicity when compared with its predecessor. There is an ongoing phase I/II trial to determine MTD in adult patients with relapsed or refractory ALL (26). Similar to combotox, capillary leak was a dose limiting toxicity.
SGN CD19A—denintuzumab mafodotin
Denintuzumab mafodotin is a monoclonal antibody targeting CD19 that is conjugated to monomethylauristatin F (MMAF), an agent that binds and disrupts microtubules. This antibody-drug conjugate is internalized upon binding to CD19 on the surface of cells and releases MMAF, which binds to tubulin. This eventually leads to cell cycle arrest and apoptosis. A phase I dose escalation study is being performed in patients with aggressive relapsed or refractory B cell lymphoma, Burkitt’s lymphoma and relapsed or refractory ALL. A total of 92 patients have been enrolled in this study since 2013, and this study is expected to complete in 2018. The primary objective of this study has been to determine the maximal tolerated dose, starting at 0.3 mg/kg up to 6 mg/kg (NCT01786096).
SAR 3419—coltuximab ravtansine
Coltuximab ravtansine is a monoclonal antibody against CD19 conjugated with a maytansinoid compound that binds tubulin and exerts its effect in a manner similar to the vinka alkaloids. However, maytansinoid compounds are generally considered to be more potent than vinka alkaloids. A phase I study has been performed in B cell lymphomas to determine the maximal tolerated dose, and additional phase II studies are also ongoing as well. Phase II studies have since also been performed in adults with relapsed ALL. While the drug was well tolerated, there was a poor clinical response with only a 25% ORR and duration of response of only 1.9 months (27). The most prominent adverse effects included diarrhea, nausea, and fever. There have also been reports of reversible vision loss associated with changes in corneal epithelium.
Blinatumomab
Blinatumomab is a bispecific T cell engaging antibody that acts by redirecting cytotoxic T cells to cells expressing CD19. The antibody itself contains the variable domains of CD19 and CD3, which are linked together. Once bound to CD19 as part of the antibody complex, cytotoxic T cells induce cell death via the perforin system. Blinatumomab is an FDA approved drug for relapse and refractory ALL.
Due to its short half-life, blinatumomab is given as a continuous infusion over a prolonged period of time. Associated adverse reactions include fever, headache, neutropenia, and edema. Encephalopathy and cytokine release syndrome (CRS) have been documented as well. Most studies to date with blinatumomab have investigated outcomes in patients who have MRD or relapsed or refractory ALL.
In the initial studies, blinatumomab was used in patients with MRD, who were at risk for relapse. Twenty patients with MRD positivity were treated with blinatumomab as a continuous infusion for a total daily dose of 15 µg/kg for 28 days, every 6 weeks. After one cycle, patients were able to proceed with allogeneic stem cell transplantation or continue on therapy with blinatumomab for up to three additional cycles. After one cycle, 16 of 20 patients treated with blinatumomab were found to have converted to negative MRD (28). On long-term follow-up of these 20 patients, 61% had relapse free survival (RFS) at 33 months, in comparison to 42% RFS in historical controls (29).
Blinatumomab was also studied in the relapsed and refractory setting in two large phase II trials. The German ALL group studied the use of blinatumomab in 36 patients with relapsed or refractory ALL. Of those patients, 42% had undergone stem cell transplantation previously. The overall response rate was found to be 69% within two cycles of therapy, and 88% of those who responded had negative MRD (30). An additional study was then performed evaluating 189 patients with high risk disease and had a CR of 43% (31). Blinatumomab is being studied in a phase III randomized clinical trial in first relapse ALL patients randomized to blinatumomab versus combination chemotherapy (NCT02101853). In addition, there is a phase III randomized clinical trial investigating the use of standard chemotherapy against chemotherapy plus blinatumomab in the upfront setting (NCT02003222).
CAR T cells
Background
CAR T cells are autologous T cells that are re-engineered to specifically exert cytotoxic effects on cancer cells. While it would be ideal to engineer a CAR T cell targeted specifically towards leukemic cells, there is no known ubiquitous antigen that is solely expressed on malignant B cells and not on normal cells. CD19 however, represents an ideal option as it is expressed in most B cell malignancies with off tumor expression limited to mature B cells. To date, most clinical trials utilizing anti-CD19 directed CAR T cells have used autologous T cells that are initially pheresed from the patient, genetically engineered ex-vivo then re-infused into the patient after they receive lymphodepleting chemotherapy (Table 1).
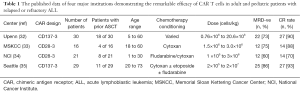
Full table
Anti-CD19 CARs consist of an antibody single chain variable fragment targeting CD19, bound to a hinge and linker molecule that traverses the cell membrane connecting to the CD3 intracellular signaling domain with the addition of co-stimulatory domains. The most commonly used co-stimulatory domains in CART clinical trials are CD28 and 4-1BB. Investigations are ongoing to further optimize the CAR construct to yield more potent activity and longer persistence.
Efficacy
There have now been several large single center phase 2 trials showing the unprecedented efficacy of anti-CD19-CAR T cells in adult and pediatric patients with relapsed or refractory ALL (32-37).
A cohort of 30 adult and pediatric patients treated with anti-CD19-CAR containing the 4-1BB construct from the University of Pennsylvania was studied. Complete response was achieved in 90% of patients (32). An additional clinical trial at Memorial Sloan Kettering Cancer Center (MSKCC) evaluated 16 patients with relapsed ALL treated with anti-CD19 CAR T cells with the CD28 co-stimulatory domain. Complete response was achieved in 88% of patients (33). The National Cancer Institute (NCI) treated 21 patients with CD19-28 CAR therapy who had relapsed disease and had a complete response rate of 70% (34). Lastly, a study at Fred Hutchinson Cancer Research Center was recently published in which 29 patients with relapsed ALL were treated with CD19-4-1BB CAR therapy and obtained a complete response rate of 93% (35).
Anti-CD19 CAR T cells have a tremendous initial response rate, but many patients still relapse (38). With the loss of CAR T cell persistence, CD19+ relapses can occur. In addition, relapses can occur with the emergence of CD19 negative disease. A mechanism of immune escape involving a variety of missense and de novo mutations allow B cells to express a truncated CD19 variant not recognized by CD19 CAR T cells (39).
Anti-CD22 CAR T cells have already been developed and have shown efficacy in animal models (40). CD22 is an optimal target similar to CD19 in that it is also ubiquitously expressed throughout the various stages of the B cell lineage. There are clinical trials currently investigating the use of CD22 CAR T cells in adult and pediatric patients with relapsed or refractory B cell ALL. One such trial is being performed at the NCI with an expected enrollment of 57 pediatric and young adult patients (NCT02315612). There are also ongoing phase I trials at the University of Pennsylvania studying the safety and efficacy of CD22-4-1BB CAR T cells in relapsed or refractory ALL in the adult population (NCT02588456) as well as the pediatric and young adult population (NCT02650414). In addition, CAR T cells directed against thymic stromal lymphoprotein receptor (TSLPR), which is over-expressed in a small subset of ALL and confers a poor prognosis are also in development and have shown activity in animal models (41).
While CAR therapy has shown striking activity and significant promise, serious treatment related adverse effects including CRS and neurotoxicity can occur (32-35). Focuses of pre-clinical and clinical investigations are to maintain response rates while mitigating potential treatment related toxicity.
Toxicity
CRS is a potentially fatal adverse effect of CAR T cell therapy and other T cell engagers such as blinatumomab. There is a clear correlation between the incidence and severity of CRS with disease burden. The pathogenesis of CRS is an exaggerated inflammatory response in the setting of T cell activation and proliferation, resulting in significantly elevated chemokine signaling. Symptoms commonly consist of myalgia and fever, but shock and multi-organ failure can also occur. Some patients may eventually need ICU level care with respiratory and vasopressor support. Elevated levels of IL-2, IL-6, IL-10 and interferon have been seen and can predict the incidence of CRS (32,35). Ferritin is also significantly elevated to levels seen in macrophage activation syndrome.
Of all the pro-inflammatory cytokines that could be targeted to mitigate the effect of CRS, disrupting IL-6 seems to be most efficacious (32). Tocilizumab is a monoclonal antibody that binds the IL-6 receptor and it has been the most widely used anti IL-6 therapy for treatment of CRS (42). The treatment and prevention of CRS in a way that maintains high response rates is an active area of investigation in CAR T cell therapy. Potential approaches include, pre-emptive use of anti-cytokine directed therapy, real time dose adjustment in response to early CRS toxicity through a fractionated dosing scheme (43) and an inverse dose/disease burden-based approach (35,44).
Encephalopathy and seizures are some of the CNS adverse events reported in CAR T cell therapy (32,35). These events have occurred after febrile episodes or CRS, and they have generally been self-limiting. The etiology behind this effect has not been well described by any laboratory or imaging modality.
B cell aplasia is an expected side effect of CAR T cell therapy as CD19 is almost universally expressed on B cells (45,46). Often, the presence of hypogammaglobulinemia and B cell aplasia serves as a marker of CAR T cell persistence and activity. Intravenous immunoglobulin supplementation is usually helpful in preventing significant infectious complications related to B cell aplasia, but this has not been studied in the long-term setting.
Conclusions
Treatment options for patients with relapsed and refractory ALL are rapidly expanding with the advent of promising immunotherapy to treat this disease. In addition, the successes of these immunologic approaches in relapsed disease have driven the design of studies to incorporate this modality of treatment as part of upfront treatment paradigms. Optimizing these agents with combination approaches (either with conventional treatment or other targeted agents), and approaches to minimize toxicity will further improve the therapeutic potential of immunotherapy for ALL.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Marin Feldman Xavier) for the series “Advances on Clinical Immunotherapy” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.01.12). The series “Advances on Clinical Immunotherapy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013 CA Cancer J Clin 2013;63:11-30; 30. [Crossref] [PubMed]
- Möricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia 2010;24:265-84. [Crossref] [PubMed]
- Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol 2012;30:1663-9. [Crossref] [PubMed]
- Schrappe M, Hunger SP, Pui CH, et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med 2012;366:1371-81. [Crossref] [PubMed]
- Kantarjian H, Thomas D, O'Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer 2004;101:2788-801. [Crossref] [PubMed]
- Thomas X, Boiron JM, Huguet F, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol 2004;22:4075-86. [Crossref] [PubMed]
- Maury S, Chevret S, Thomas X, et al. Addition of Rituximab Improves the Outcome of Adult Patients with CD20-Positive, Ph-Negative, B-Cell Precursor Acute Lymphoblastic Leukemia (BCP-ALL): Results of the Randomized Graall-R 2005 Study. Blood 2015;126:1. [PubMed]
- Stock W, Yu D, Sanford B, et al. Incorporation of Alemtuzumab into Front-Line Therapy of Adult Acute Lymphoblastic Leukemia (ALL) Is Feasible: A Phase I/II Study from the Cancer and Leukemia Group B (CALGB 10102). Blood 2005;106:145.
- Raponi S, De Propris MS, Intoppa S, et al. Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: analysis of 552 cases. Leuk Lymphoma 2011;52:1098-107. [Crossref] [PubMed]
- Thomas DA, O'Brien S, Faderl S, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol 2010;28:3880-9. [Crossref] [PubMed]
- Hoelzer D, Huettmann A, Kaul F, et al. Immunochemotherapy with Rituximab Improves Molecular CR Rate and Outcome In CD20+ B-Lineage Standard and High Risk Patients; Results of 263 CD20+ Patients Studied Prospectively In GMALL Study 07/2003. Blood 2010;116:170.
- Robak T, Robak E. New anti-CD20 monoclonal antibodies for the treatment of B-cell lymphoid malignancies. BioDrugs 2011;25:13-25. [Crossref] [PubMed]
- Wierda WG, Padmanabhan S, Chan GW, et al. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood 2011;118:5126-9. [Crossref] [PubMed]
- Jabbour E, Hagop K, Thomas D, et al. Phase II Study Of The Hyper-CVAD Regimen In Combination With Ofatumumab As Frontline Therapy For Adults With CD-20 Positive Acute Lymphoblastic Leukemia (ALL). Blood 2013;122:2664. [PubMed]
- Raetz EA, Cairo MS, Borowitz MJ, et al. Re-induction chemoimmunotherapy with epratuzumab in relapsed acute lymphoblastic leukemia (ALL): Phase II results from Children's Oncology Group (COG) study ADVL04P2. Pediatr Blood Cancer 2015;62:1171-5. [Crossref] [PubMed]
- Advani AS, McDonough S, Coutre S, et al. SWOG S0910: a phase 2 trial oflofarabine/cytarabine/epratuzumab for relapsed/refractory acute lymphocytic leukaemia. Br J Haematol 2014;165:504-9. [Crossref] [PubMed]
- Stock W, Sanford B, Lozanski G, et al. Alemtuzumab can be incorporated into front-line therapy of adult acute lymphoblastic leukemia (ALL): Final phase I results of a Cancer and Leukemia Group B study (CALGB 10102) Blood 2009;114:838.
- Angiolillo AL, Yu AL, Reaman G, et al. A phase II study of Campath-1H in children with relapsed or refractory acute lymphoblastic leukemia: a Children's Oncology Group report. Pediatr Blood Cancer 2009;53:978-83. [Crossref] [PubMed]
- Kebriaei P, Saliba RM, Ma C, et al. Allogeneic Transplantation after an Alemtuzumab-Containing Myeloablative Conditioning Regimen for CD52 Positive Acute Lymphoblastic Leukemia (ALL). Blood 2005;106:1135. [PubMed]
- Gorin NC, Isnard F, Garderet L, et al. Administration of alemtuzumab and G-CSF to adults with relapsed or refractory acute lymphoblastic leukemia: results of a phase II study. Eur J Haematol 2013;91:315-21. [PubMed]
- Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol 2012;13:403-11. [Crossref] [PubMed]
- Kantarjian H, Thomas D, Jorgensen J, et al. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer 2013;119:2728-36. [Crossref] [PubMed]
- Jabbour E, O'Brien S, Sasaki K, et al. Frontline Inotuzumab Ozogamicin in Combination with Low-Intensity Chemotherapy (mini-hyper-CVD) for Older Patients with Acute Lymphoblastic Leukemia (ALL). Blood 2015;126:83.
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med 2016;375:740-53. [Crossref] [PubMed]
- Schindler J, Gajavelli S, Ravandi F, et al. A phase I study of a combination of anti-CD19 and anti-CD22 immunotoxins (Combotox) in adult patients with refractory B-lineage acute lymphoblastic leukaemia. Br J Haematol 2011;154:471-6. [Crossref] [PubMed]
- Möricke A, Reiter A, Zimmermann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 2008;111:4477-89. [Crossref] [PubMed]
- Rowe JM, Buck G, Burnett AK, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood 2005;106:3760-7. [Crossref] [PubMed]
- Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011;29:2493-8. [Crossref] [PubMed]
- Gökbuget N, Dombret H, Bonifacio M, et al. Long-Term Outcomes after Blinatumomab Treatment: Follow-up of a Phase 2 Study in Patients (Pts) with Minimal Residual Disease (MRD) Positive B-Cell Precursor Acute Lymphoblastic Leukemia (ALL). Blood 2015;126:680.
- Topp MS, Gökbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol 2014;32:4134-40. [Crossref] [PubMed]
- Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015;16:57-66. [Crossref] [PubMed]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25 [Crossref] [PubMed]
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517-28. [Crossref] [PubMed]
- Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016;126:2123-38. [Crossref] [PubMed]
- Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5:177ra38 [Crossref] [PubMed]
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509-18. [Crossref] [PubMed]
- Maude SL, Teachey DT, Rheingold SR, et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. J Clin Oncol 2016;34:abstr 3011.
- Sotillo E, Barrett DM, Black KL, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov 2015;5:1282-95. [Crossref] [PubMed]
- Haso W, Lee DW, Shah NN, et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 2013;121:1165-74. [Crossref] [PubMed]
- Qin H, Cho M, Haso W, et al. Eradication of B-ALL using chimeric antigen receptor-expressingT cells targeting the TSLPR oncoprotein. Blood 2015;126:629-39. [Crossref] [PubMed]
- Barrett DM, Teachey DT, Grupp SA. Toxicity management for patients receiving novel T-cell engaging therapies. Curr Opin Pediatr. 2014;26:43-9. [Crossref] [PubMed]
- Frey NV, Shaw PA, Hexner EO, et al. Optimizing chimeric antigen receptor (CAR) T cell therapy for adult patients with relapsed or refractory (r/r) acute lymphoblastic leukemia (ALL). J Clin Oncol 2016;34:abstr 7002.
- Park JH, Riviere I, Wang X, et al. Impact of disease burden on long-term outcome of 19-28z CAR modified T cells in adult patients with relapsed B-ALL. J Clin Oncol 2016;34:abstr 7003.
- Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor transduced T cells. Blood 2012;119:2709-20. [Crossref] [PubMed]
- Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015;7:303ra139 [Crossref] [PubMed]


