Clinicopathological features and prognosis of younger patients with gastric carcinoma
Introduction
Gastric carcinoma (GC) is the fourth most prevalent type of cancer with a low survival rate, and therefore, it places an enormous burden on societies worldwide (1,2). GC occurs most frequently in the 50–70 year age group (2-5). Many studies have shown that younger GC patients (≤50 years old) constitute a different clinical entity with its own specific clinicopathological features (6,7). Although young people are less commonly affected by GC, researchers have proposed that young patients show a worse prognosis than older patients. Specifically, GC has demonstrated more aggressive behaviour in patients under 50 years of age than in those over 50 years of age, with a median survival duration of 11 to 16 months versus 33 months, respectively (8). However, recent studies have suggested that younger GC patients have a better prognosis than older GC patients (6), and the number of studies performed on young GC patients is increasing. However, the impact of younger age (≤50 years) on the presentation and survival outcomes of GC patients remains controversial (3,6-10). Because of this lack of data on the biological and clinicopathological features of GC in young patients, we selected 487 patients with GC from one surgical team and performed a retrospective cohort study to identify the reasons for the different biological behaviour and prognosis in younger patients. The objective of this study was to assess the clinicopathological features and prognosis of GC patients younger than or equal to 50 years of age compared with the more typical GC population of older patients (51–70 years old).
Methods
Patients
From June 2009 to August 2011, 1,704 patients with GC underwent stomach surgery at Fudan University Shanghai Cancer Center. Four hundred and sixty-three patients underwent surgeries performed by the same chief surgeon. All patients were diagnosed using barium meal, endoscopy, and pathological examination. Clinical data were acquired from the medical records through our computerised documentation system (ChiBASE) and telephone investigation. Four hundred and eleven of the patients were chosen and categorized into the following two groups: the young group, YG (≤50 years old, 102 patients), and the old group, OG (51–70 years old, 290 patients). Seventy-one patients older than 70 years of age were excluded. The inclusion criteria consisted of the following: (I) patients with GC who were pathologically confirmed; (II) patients who underwent radical or palliative surgeries without previous gastric surgery or neoadjuvant chemotherapy; (III) patients who died from other diseases (not GC or related to GC) were excluded. This study was approved by the local ethic committee, and the clinical features analysed included patient age, pathogenesis prior to hospital visit, gender, tumor size, tumor location, histological grade, tumor-node-metastasis (TNM) stage, Borrmann type, anaemia, angiolymphatic invasion, carcinoembryonic antigen (CEA) level, carbohydrate antigen 19-9 (CA19-9) level, surgical approach and survival.
Follow-up
The follow-up for all patients was regularly performed by telephone or periodic review, and 93.4% of patients received full follow-up. The follow-up period was defined as beginning from the date of treatment to the date of death or final follow-up. The final follow-up date was September 1, 2015.
Evaluation
Pathogenesis prior to hospital visit was defined as ranging from the time alarm symptoms developed to the time at which patients visited the doctor. The TNM classification of GC stage was based on the 7th edition of TNM staging (UICC 2009). Surgical and clinicopathological features were recorded on the basis of the Japanese Classification of Gastric Carcinoma. The following two histological types were included: differentiated (papillary adenocarcinoma and well/moderately differentiated adenocarcinomas) and undifferentiated (poorly or undifferentiated adenocarcinoma, mucinous carcinoma or signet ring cell). For calculation of the survival curves and 5-year disease-specific survival rate, patients who did not undergo tumor resection were excluded because they lacked complete histopathological examination or staging.
Statistical analysis
Statistical analysis was performed using SPSS version 21.0 statistical software (SPSS IBM USA). The figures were constructed using GraphPad Prism 5.0 (La Jolla, CA). The data are presented as the mean ± SD. The chi square test and independent T test were used to compare the clinicopathological data. The Kaplan-Meier method was used to calculate the OS rates. The log-rank test was used for the univariate analysis of the relationship between the clinical features and prognosis. Multivariate cox proportional hazards models were used when meaningful factors (P<0.05 in univariate analysis) and features with prognostic value were selected to identify the clinicopathological features that were independent predictive markers of the YG. P<0.05 was considered statistically significant for all methods.
Results
Demographic distribution and clinicopathological features
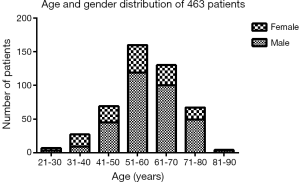
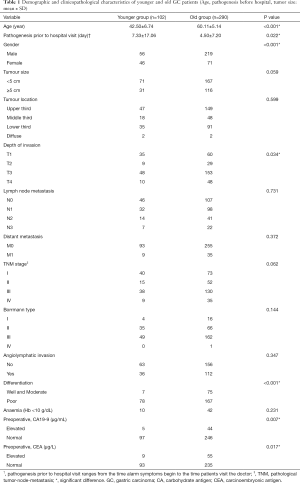
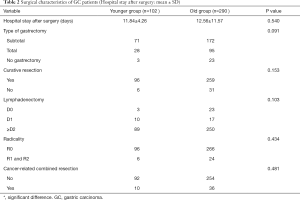
The demographic distribution and clinicopathological features of all subjects enrolled in this study are summarized in Figure 1 and Table 1. The YG consisted of 102 patients ≤50 years of age, while the OG consisted of the remaining 290 patients (>50 and ≤70 years of age). In the YG, 56 patients were male (54.9%) and 46 patients were female (45.1%), while in the OG, 219 were male (75.5%) and 71 were female (24.5%). The age and gender distributions are shown in Figure 1. Most patients were 51–70 years old, accounting for 74.0% of all patients. The male-to-female ratio of all GC patients was 2.35:1, but it was 1.22:1 in the YG and 3.08:1 in the OG. The mean age was significantly different in the two study groups at 42.50±6.74 years in the YG versus 60.11±5.14 years in the OG (P<0.001). Regarding time when alarm symptoms began, the YG remained untreated for a significantly longer duration in comparison to the OG (7.33±17.06 days versus 4.50±7.20 days, respectively; 0.022), indicating the YG received a more delayed diagnosis. The YG also differed significantly from the OG in terms of a higher percentage of the T1 depth of invasion (0.034), undifferentiated histological grade (P<0.001), normal preoperative CA19-9 level (P=0.07) and normal preoperative CEA level (P=0.017). However, no significant differences were found between the two groups in tumor size (P=0.059), tumor location (P=0.599), TNM stage (P=0.062), Borrmann type (P=0.144), lymph node metastasis (P=0.731), distant metastasis (P=0.372), angiolymphatic invasion (P=0.347) and anaemia (P=0.231). Table 2 shows the surgical features of the GC patients. The YG had a shorter hospital stay after surgery but without significance (11.84±4.26 vs. 12.56±11.57 days; P=0.540), and there were no significant differences for the other surgical features between the two groups.
Full table
Full table
Survival and prognostic factor
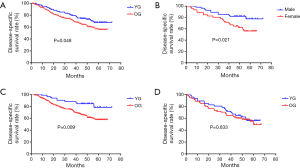
As of September 1, 2015, the 5-year disease-specific survival rate for all 302 patients was 51.3%. The median observation periods were 46.4 months in the YG (range, 4–72 months) versus 43.1 months in the OG (range, 1–68 months). Thirty of our younger patients died during the study period. The 5-year disease-specific survival rate was 67.7% in the YG and 55.8% in the OG. The survival curves of the two groups are shown in Figure 2A. The 5-year disease-specific survival rate of the YG was better than that of the OG (P=0.048, Figure 2A). In the YG, a significantly lower survival rate was observed for female patients with GC compared to males with GC, as presented in Figure 2B (56.1% and 77.6%, respectively; P=0.021). Male patients with GC in the YG showed a significantly higher 5-year disease-specific survival rate compared to those in the OG (77.6% and 57.6%, respectively; P=0.009; Figure 2C), while females did not present this difference with age (56.1% and 49.4%, respectively; P=0.633; Figure 2D).
Of all 109 younger GC patients analysed in this study, univariate analysis showed that the tumor size, depth of invasion, lymph node involvement, distant metastatic spread, Lymphatic infiltration, venous invasion, CA19-9 level and sex were prognostic factors (Table 3). Furthermore, multivariate analysis using Cox regression demonstrated that the tumor size (≥5 cm), N classification (N2/3), elevated CA19-9 level and sex (Female) were independent negative prognostic factors of GC patients aged ≤50 years old (Table 3). In contrast, T classification (T3/4) and N classification (N2/3) were independent negative prognostic factors of GC patients aged >50 years old (P=0.023, P=0.001, respectively, Table 4) and sex (female) had no effect on their prognosis (P=0.370, Table 4).

Full table

Full table
TNM stage distribution
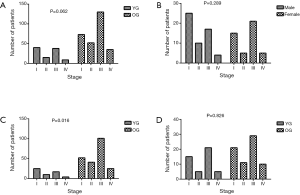
The TNM stage distribution was compared between the YG and OG, males and females in the YG, males in the YG and OG, and females in the YG and OG (Figure 3). More patients were in the I-stage and fewer patients were in the III-stage in the YG than the OG, but this finding was not significant (39.2% and 37.3% vs. 25.2% and 44.8%, respectively; Figure 3A, P=0.062). In the YG, males showed a higher proportion of I-stage disease than females, but this finding also was not significant (44.6% vs. 32.6%, respectively; Figure 3B, P=0.289). In addition, no significant difference was found between the TNM stage distribution of the YG and OG in females (Figure 3D, P=0.826). Figure 3C shows that there was a significant difference between the YG and OG among males; in particular, males in the YG showed a higher percentage of I-stage and a lower percentage of III-stage compared to males in the OG (44.6% and 30.4% vs. 23.7% and 46.1%, respectively; P=0.016).
Discussion
GC is generally considered a disease of the elderly, and patients have a mean age of over 60 years at diagnosis (10,11). GC occurs most frequently in the 50–70 year age group (2-5). However, the rate of GC in young patients has increased in recent decades despite a reduction in the overall prevalence of the disease (12-14). Due to incomplete data regarding whether GC in younger patients differs from that in older patients, we focused on patients ≤50 years old. In particular, we retrospectively analysed 392 patients with GC who underwent a stomach resection operation performed by the same surgeon, including 102 (26.0%) patients in the YG and 290 (74.0%) in the OG.
Previous studies have demonstrated numerous clinical differences between younger and old patients with GC. In particular, these results showed that the percentage of females was higher among younger compared to old patients (8,15-19), and our findings are consistent with these reports (P<0.001). Several reports have also suggested that hormonal factors might account for the higher percentage of young females with GC. Younger females show a higher level of oestrogen and an increased proportion of oestrogen receptor-positive cells (19-21). Nevertheless, the correlation between gender hormones and GC prognosis remains controversial, and further research is needed to confirm whether gender impacts the prognosis of younger patients.
According to our data, there were higher proportions of cases with an undifferentiated histological grade and the signet ring cell type of GC in younger patients, which is consistent with previous studies showing that younger patients display poorly differentiated histology (4,6,8,22). However, there were no significant differences in the tumor size, tumor location, TNM stage, Borrmann type, lymph node metastasis, distant metastasis, angiolymphatic invasion, and anaemia between the two groups in our study. The YG had a longer latency before presenting to the hospital than the OG, indicating that younger patients lack awareness of their self-health, which may lead to delayed diagnosis. Additionally, there were no significant differences in surgical features between the groups, such as the type of gastrectomy, curative resection, lymphadenectomy, radicality, and cancer-related combined resection. Though no significant difference was found between two groups, the comparatively shorter hospital stay after surgery for the YG might indicate that younger patients were more able to withstand stomach resection surgery.
The higher percentage of T1 depth of invasion, early stage and good physical fitness might explain the good outcome of the YG. In addition, the aggressive histology of younger GC patients may be balanced by the comparatively higher percentage of I/II TNM stage distribution. A higher percentage of patients with a normal CA19-9 level was also associated with the improved survival observed in the YG. Angiolymphatic invasion has been reported to be a negative factor associated with distant metastasis (23), and the YG showed a lower frequency of angiolymphatic invasion than the OG, but the difference lacked significance (P=0.347).
The 5-year disease-specific survival rate in the YG was significantly higher than that in the OG (P=0.048), which is consistent with the results of recent studies (6,7). In the YG, male patients showed a significantly higher survival rate than females (P=0.021). The 5-year disease-specific survival rate of male patients with GC in the YG was significantly higher than that of male patients in the OG (P=0.009), while the 5-year disease-specific survival of females was not different between age groups (P=0.633). These findings indicated that the better prognosis of the YG was most likely due to the higher survival rate among males than females. The lower TNM stage distribution of males in the YG and more advanced TNM stage of males in the OG may partially explain the good prognosis of males in the YG.Younger patients, especially males, diagnosed in earlier stage might be associated with their positive attitude and convenience to hospital. So, male GC patients under 50 years of age showed an improved 5-year survival rate. Additionally, the prognosis of females was poor for both the YG and OG, with no significant difference related to age. A previous study is in agreement with our findings (24); however, there is no precise explanation for these similarities. Furukawa et al. (25) suggested that females’ analogues or sex hormones might affect carcinogenesis or stomach cancer progression. In addition, pregnancy and delivery may accelerate the growth of stomach cancer cells, although the exact molecular mechanism responsible for this effect remains unclear.
Younger patients show distinguishing features and prognostic results compared to elderly patients; therefore, exploring and evaluating the prognostic factors that influence the survival rate in younger patients is meaningful. The prognosis for females was worse than that for males in the YG. In the present study, we also found that tumor size (≥5 cm), N classification (N2/3), elevated CA19-9 level and sex (Female) served as independent negative prognostic factors for younger patients with GC. Similarly, the CA19-9 level was previously reported to be a negative prognostic indicator (26), which is consistent with our findings.
It was the first time to exclude the factor of surgeon’s skill and we believe our results might provide meaningful conclusions regarding younger patients with GC. However, this was a small, single-centre study which lack in-depth study of the mechanism. Further large sample study with in-depth exploration of the mechanism was needed.
In summary, GC patients ≤50 years of age showed distinct features, including a higher percentage of females, a higher percentage of the T1 depth of invasion, undifferentiated histological grade, normal preoperative CA19-9 level and normal preoperative CEA level in comparison to the OG (51–70 years of age). Males ≤50 years of age more commonly showed early staged disease with better survival, while the prognosis of females ≤50 years of age was as poor as that of females >50 years of age. Thus, enhancing young people’s awareness of self-health is necessary, particularly for young females.
Acknowledgments
The authors acknowledge the work of Yuezhen Dai.
Funding: This study was supported by grants from the Natural Science Foundation of China (No. 81272726).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.19). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethic committee of Fudan university cancer center and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [Crossref] [PubMed]
- Nakamura R, Saikawa Y, Takahashi T, et al. Retrospective analysis of prognostic outcome of gastric cancer in young patients. Int J Clin Oncol 2011;16:328-34. [Crossref] [PubMed]
- Smith BR, Stabile BE. Extreme aggressiveness and lethality of gastric adenocarcinoma in the very young. Arch Surg 2009;144:506-10. [Crossref] [PubMed]
- Saito H, Takaya S, Fukumoto Y, et al. Clinicopathologic characteristics and prognosis of gastric cancer in young patients. Yonago Acta Med 2012;55:57-61. [PubMed]
- Dhobi MA, Wani KA, Parray FQ, et al. Gastric cancer in young patients. Int J Surg Oncol 2013;2013:981654 [Crossref] [PubMed]
- Qiu MZ, Wang ZQ, Zhang DS, et al. Clinicopathological characteristics and prognostic analysis of gastric cancer in the young adult in China. Tumour Biol 2011;32:509-14. [Crossref] [PubMed]
- Schildberg CW, Croner R, Schellerer V, et al. Differences in the treatment of young gastric cancer patients: patients under 50 years have better 5-year survival than older patients. Adv Med Sci 2012;57:259-65. [Crossref] [PubMed]
- Pisanu A, Podda M, Cois A, et al. Gastric cancer in the young: is it a different clinical entity? A retrospective cohort study. Gastroenterol Res Pract 2014;2014:125038 [Crossref] [PubMed]
- Park JC, Lee YC, Kim JH, et al. Clinicopathological aspects and prognostic value with respect to age: an analysis of 3,362 consecutive gastric cancer patients. J Surg Oncol 2009;99:395-401. [Crossref] [PubMed]
- Ramos-De la Medina A, Salgado-Nesme N, Torres-Villalobos G, et al. Clinicopathologic characteristics of gastric cancer in a young patient population. J Gastrointest Surg 2004;8:240-4. [Crossref] [PubMed]
- Grabiec J, Owen DA. Carcinoma of the stomach in young persons. Cancer 1985;56:388-96. [Crossref] [PubMed]
- Mitsudomi T, Matsusaka T, Wakasugi K, et al. A clinicopathological study of gastric cancer with special reference to age of the patients: an analysis of 1,630 cases. World J Surg 1989;13:225-30; discussion 230-1. [Crossref] [PubMed]
- Bai Y, Li ZS. Endoscopic,clinicopathological features and prognosis of very young patients with gastric cancer. J Gastroenterol Hepatol 2011;26:1626-9. [Crossref] [PubMed]
- Kong X, Wang JL, Chen HM, et al. Comparison of the clinicopathological characteristics of young and Elderly patients with gastric carcinoma: a meta analysis. J Surg Oncol 2012;106:346-52. [Crossref] [PubMed]
- Matley PJ, Dent DM, Madden MV, et al. Gastric carcinoma in young adults. Ann Surg 1988;208:593-6. [Crossref] [PubMed]
- Eguchi T, Takahashi Y, Yamagata M, et al. Gastric cancer in young patients. J Am Coll Surg 1999;188:22-6. [Crossref] [PubMed]
- Kim DY, Ryu SY, Kim YJ, et al. Clinicopathological characteristics of gastric carcinoma in young patients. Langenbecks Arch Surg 2003;388:245-9. [Crossref] [PubMed]
- Santoro R, Carboni F, Lepiane P, et al. Clinicopathological features and prognosis of gastric cancer in young European adults. Br J Surg 2007;94:737-42. [Crossref] [PubMed]
- Lai JF, Kim S, Li C, et al. Clinicopathologic characteristics and prognosis for young gastric adenocarcinoma patients after curative resection. Ann Surg Oncol 2008;15:1464-9. [Crossref] [PubMed]
- Isobe T, Hashimoto K, Kizaki J, et al. Characteristics and prognosis of gastric cancer in young patients. Oncol Rep 2013;30:43-9. [PubMed]
- Kojima O, Takahashi T, Kawakami S, et al. Localization of estrogen receptors in gastric cancer using immunohistochemical staining of monoclonal antibody. Cancer 1991;67:2401-6. [Crossref] [PubMed]
- Park HJ, Ahn JY, Jung HY, et al. Clinical characteristics and outcomes for gastric cancer patients aged 18–30 years. Gastric Cancer 2014;17:649-60. [Crossref] [PubMed]
- Setälä LP, Kosma VM, Marin S, et al. Prognostic factors in gastric cancer: the value of vascular invasion, mitotic rate and lymphoplasmacytic infiltration. Br J Cancer 1996;74:766-72. [Crossref] [PubMed]
- Kim JH, Boo YJ, Park JM, et al. Incidence and long term outcome of young patients with gastric carcinoma according to sex. Arch Surg 2008;143:1062-7; discussion 1067. [Crossref] [PubMed]
- Furukawa H, Iwanaga T, Hiratsuka M, et al. Gastric cancer in young adults:growth accelerating effect of pregnancy and delivery. J Surg Oncol 1994;55:3-6. [Crossref] [PubMed]
- Qiu MZ, Lin JZ, Wang ZQ, et al. Cutoff value of carcinoembryonic antigen and carbohydrate antigen 19-9 elevation levels for monitoring recurrence in patients with resectable gastric adenocarcinoma. Int J Biol Markers 2009;24:258-64. [Crossref] [PubMed]