
Neoadjuvant chemoradiotherapy versus chemotherapy and surgery for patients with locally advanced esophageal squamous cell carcinoma
Introduction
Esophageal carcinoma is the eighth most common type of cancer worldwide, with estimated 455,800 new cases diagnosed and 400,200 mortalities in 2012. Esophageal adenocarcinomas (AC) are relatively common in western countries, while 90% of cases are squamous cell carcinomas (SCC) in Eastern countries, especially in China (1). For patients with locally advanced esophageal carcinoma, surgery remains to be the main therapeutic strategy, but a part of patients will experience relapse within 2 years after resection. Neoadjuvant chemoradiotherapy (nCRT) or neoadjuvant chemotherapy (nCT) has been shown to improve survival for locally advanced esophageal carcinoma (2-4). Compared with surgery alone, nCRT achieved a better 5-year OS for locally advanced esophageal carcinoma (47% vs. 34%; P=0.003) and patients with SCC benefit more (2). In a recent meta-analysis, Sjoquist et al. confirmed significant survival benefit from neoadjuvant CRT and, to a lesser extent, neoadjuvant CT in patients with SCC or AC of the esophagus (5). Further study indicated that nCRT was superior to nCT in patients with esophageal AC and the 3-year survival rate improved from 27.7% to 47.4% by the addition of neoadjuvant radiation therapy. However, the trial was closed early and statistical significance was not achieved (6). To the best of our knowledge, there is limited data available to support which kind of neoadjuvant therapy is better for patients with esophageal squamous cell carcinoma (ESCC). Neoadjuvant CRT followed by surgery has been recommended as the standard treatment regardless of the histological type. However, in China nCT rather than nCRT is preferred for a large cohort of patients with locally advanced esophageal carcinoma to reduce the odds of postoperative morbidity. In this study, we retrospectively investigated clinical efficacy and postoperative morbidity of nCRT and nCT in patients with locally advanced ESCC who underwent subsequent esophagectomy.
Methods
Patients
Based on a prospective institutional database at the Department of Oncology of Zhengzhou University Affiliated Cancer Hospital from January 2009 to January 2014, locally advanced esophageal carcinoma patients who received nCRT or chemotherapy at our institution were enrolled in this study. All patients met the following inclusion criteria: (I) histologically proven SCC; (II) clinical stage T2-4aN0-1M0 based on the 6th UICC-TNM classification; (III) resectable disease; (IV) 18 to 75 years of age; (V) Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1; (VI) no history of other cancer or previous radiotherapy or chemotherapy against esophageal carcinoma.
Pretreatment work-up
The pre-therapeutic included physical examination, laboratory tests, pulmonary function test, endoscopic ultrasound (EUS), barium esophagogram and contrast-enhanced computed tomography (CT) of the neck, chest and the upper abdomen. Staging was classified according to the 6th UICC-TNM classification. Lymph nodes measuring more than 10 mm were considered to be malignant by CT or EUS.
Neoadjuvant CRT
Three-dimensional conformal radiation (3D-CRT) or intensity modulated radiation therapy (IMRT) using 6MV photons were used for radiotherapy plan. A total dose of 40 Gy was delivered in 20 fractions (five fractions per week) over 4 weeks, starting on the first day of chemotherapy. The gross tumor volume is defined as the primary tumor and the metastatic lymph nodes measuring 10 mm along the short axis. The clinical target volume (CTV) includes the primary tumor with a proximal and distal of 3 cm, metastatic lymph nodes and regional lymph nodes. The regional lymph nodes include bilaterally supraclavicular fossae and superior mediastinal lymph nodes for carcinoma of the upper thoracic esophagus, mediastinal lymph nodes for carcinoma of the middle thoracic esophagus and mediastinal and perigastric lymph nodes for lower thoracic esophagus. The planning target volume is defined as CTV plus a 0.6 cm margin in the lateral direction and a 1 cm margin in the cranio-caudal direction to account for respiratory organ motion and daily set-up error.
Neoadjuvant CT consisted of cisplatin (20 mg/m2) administered intravenously on days 1 to 4 and 5-fluorouracil (5-FU) (500 mg/m2) administered intravenously on days 1 to 5. Patients received only one cycle of chemotherapy before surgery.
Neoadjuvant CT
Cisplatin plus 5-FU (DF) or Cisplatin plus paclitaxel (TP) were used in the nCT group. The DF regimen consisted of cisplatin (20 mg/m2) administered intravenously on days 1 to 4 and 5-FU (500 mg/m2) administered intravenously on days 1 to 5. The TP regimen consisted of cisplatin (20 mg/m2) administered intravenously on days 1 to 4, paclitaxel (87.5 mg/m2) administered intravenously on days 1 and 8. All patients received two courses of DF or TP before esophagectomy.
Surgery and pathological analysis
Esophagectomy with two-field lymphadenectomy was performed 2 to 3 weeks after neoadjuvant treatment. The surgery consisted of sweet procedure, Mc-Keown or minimally invasive esophagectomy (MIE), depending on tumor localization and patient characteristics. The continuity of the digestive tract was preserved with an esophagogastric end-to-side anastomosis. Resections were classified as three categories: the complete removal of the tumor, with microscopic examination of margins free of tumor cells (R0), microscopic examination of margins showing tumor cells (R1) and macroscopic examination showing tumor cells (R2). Patients with no residual viable tumor cells in the resected specimen were defined as pathologic complete response (pCR).
Postoperative CT
Patients with lymph node positive after surgery were given two courses of chemotherapy. Postoperative chemotherapy regimen was the same as preoperative one (DF or TP). Patients who showed loss of response to nCT would cross over to receive another regimen.
Follow-up
After surgery, all patients were followed every 3 months for the first 2 years and every 6 months for 3 or more years at the outpatient clinic. Physical examination, barium esophagogram and CT were used to assess recurrence. Recurrent disease was classified as locoregional or distant. Whenever a relapse was suspected, radiologic, endoscopic, or histologic confirmation was required for a diagnosis of recurrence.
Statistical analysis
Overall survival (OS) was estimated from the date of surgery to the date of death, or last follow-up. Disease-free survival (DFS) was measured from the date of surgery to first evidence of relapse or death due to any cause. DFS and OS were estimated by the Kaplan-Meier method. Continuous variables were compared using Student’s t-test (mean). Categorical variables were compared by Chi-square or Fisher exact test when appropriate. Differences were considered to be significant at the level of P<0.05. All calculations were performed with the SPSS software version 21.0 (Statistical Package for the Social Sciences, IBM Corp., Armonk, NY, USA).
Results
A total of 134 patients received neoadjuvant treatment. Ten in nCRT (1 had progress disease, 1 had poor medical condition, 8 refusal to surgery) and 13 patients (3 had progress disease, 10 refusal to surgery) in nCT, respectively, did not receive surgery. The remaining 111 patients underwent esophagectomy after completion of neoadjuvant treatment. Among them, 58 (52.3%) received nCT and 53 (47.7%) underwent nCRT. Patients and tumor characteristics are shown in Table 1. Patients were restaged with barium esophagogram and contrast-enhanced CT of the neck, chest and the upper abdomen within one week after nCRT or nCT. For nCRT group, 6 patients achieved CR, 44 achieved PR and 3 achieved SD. Of 58 patients who underwent nCT, 5 patients achieved CR, 40 achieved PR, and 13 achieved SD.
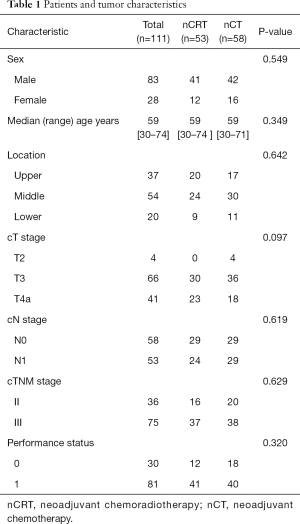
Full table
Toxicity of neoadjuvant treatment
Toxicity of neoadjuvant treatment was commonly mild. The incidences of major grade 3 or 4 adverse events in nCRT and nCT were 4 and 3 for leucopenia, 1 and 0 for esophagitis, 2 and 4 for nausea/vomiting, respectively. No patients died of complications related to neoadjuvant treatment.
Surgery and pathological analysis
Sweet procedure was performed on most patients (54.1%). MIE was only performed in the nCT group for fear of higher intraoperative risks related to radiation. The pCR rate in the nCRT group was nearly twice that of nCT group, but showed no significant differences. The proportion of patients with pN0 in nCRT group (86.8%) was more than that of nCT group (70.7%). The type of surgery and histological results are shown in Table 2.
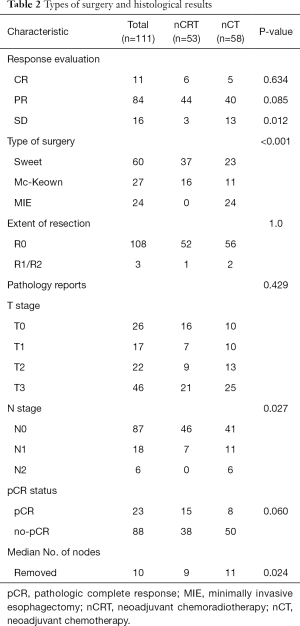
Full table
Postoperative morbidity
Postoperative morbidity was observed in 22 patients in the nCT group and 17 patients in the CRT group (P=0.660). Postoperative complications are summarized in Table 3. One patients (1.9%) in the nCRT group died within 30 days after surgery, as did one (1.7%) in the nCT group (P=1.0). Median postoperative hospital stay was 14 days (range 7 to 45 days) and 15 days (range 9 to 60 days) for nCRT and nCT group, respectively. No differences were found between the two groups (P=0.294).
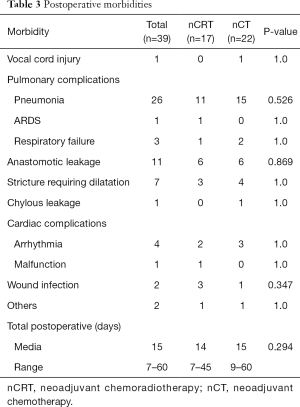
Full table
Postoperative chemotherapy
Only 11 patients (45.8%) underwent two courses and 5 (20.8%) patients underwent one course of postoperative chemotherapy. Eight patients (33.3%) did not receive postoperative chemotherapy because of postoperative complication.
Survival
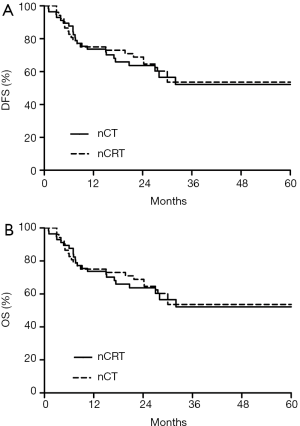
At the date of evaluation (March 2015), 21 and 18 patients had died in nCRT and nCT group, respectively. Median follow-up was 27.6 (range, 15.4 to 60.9) months for nCT group, and 47.9 (range, 16 to 74.7) months for nCRT group. The median OS and DFS of two groups were not reached. Respective DFS rates at 1, 2, and 3 years were 73.1%, 66.7%, and 53.6% in the nCRT group, compared to 73.7%, 60.4%, and 52.2% in the nCT group. Respective OS rates at 1, 2, and 3 years were 88.5%, 78.0% and 59.5% in the nCRT group, compared to 89.5%, 72.9% and 56.2% in the nCT group. DFS (P=0.848) and OS (P=0.749) were not significantly different between the two groups (Figure 1).
Subgroup analysis demonstrated that patients with clinical stage II (P=0.720) or III (P=0.867) did not benefit from nCRT. Unavailable analysis identified pN (HR =2.015, 95% CI: 1.225–3.314, P=0.006) stage as a survival factor. In the multivariable analysis, pT (HR =1.410, 95% CI: 1.053–1.888, P=0.021) and pN (HR =1.953, 95% CI: 1.717–3.257, P=0.01) stage, but not the neoadjuvant treatment type (HR =0.785, 95% CI: 0.380–1.623, P=0.514) were independent factors for survival.
Recurrence
As shown in Table 4, relapses occurred in 20 patients for the nCRT group and in 23 patients in the nCT group (P=0.836). Additional 7 patients (including 2 patients in nCRT group, and 5 in nCT group) suffering from recurrence were still alive at the end of follow-up. No significant differences were found between nCRT and nCT group in the pattern of failure.
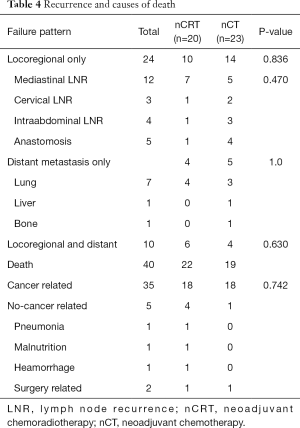
Full table
Discussion
In the past two decades, the prognosis of surgically treated esophageal carcinoma has progressively improved, not only due to precise preoperative staging, postoperative management and surgical technique, but also due to the incremental inclusion of patients with esophageal carcinoma in neoadjuvant treatment protocols (7-9). Despite these improvements, the long-term survival for patients after surgery remains poor. In our analysis, we showed that the 3-year OS was 59.5% in the nCRT group and 56.2% in the nCT group, similar to several previous trials for ESCC patients (2,10,11). The long-term result of CROSS trial showed that median OS for ESCC patients was 81.6 months in the nCRT plus surgery group and 21.1 months in the surgery alone group (12). In JOCG 9907 trial, the 5-year OS for patients with ESCC was 55% in nCT group and 43% in adjuvant chemotherapy group (11). Furthermore, OS and DFS showed no significant differences between two groups in our retrospective and nonrandomized study. Similarly, in several small sample size studies, neoadjuvant CRT did not show survival advantage compared with nCT for patients with locally advanced esophageal carcinoma, but most of these studies were on AC patients (13,14). For patients with locally advanced ESCC, it remains unclear which neoadjuvant treatment is better. JCOG1109 from Japan may resolve this problem: the partial aim of the trial was to confirm whether CRT with CDDP + 5-FU was superior to CDDP + 5-FU as preoperative therapies (15). In contrast to the trial from Japan, our study did not exclude patients with clinical stage T4, because TP showed better efficacy than DF regimen in previous reports (2,16). Thus, TP was selected as the most common nCT for patients with ESCC since 2012 at our institution. Moreover, in term of the only one cycle of chemotherapy with cisplatin and fluorouracil used in nCRT group, our prior phase II clinical trial with the same protocol in locally advanced ESCC showed that the rate of pCR was 29.5% and 5-year OS was 45.3%, consistent with the outcome of the trial from Australia for a subgroup of ESCC patients (10). Neoadjuvant CRT did not improve survival in ESCC or AC patients with clinical stage I or II compared with surgery alone (17). Interestingly, compared with patients with stage III, neoadjuvant CT with cisplatin and fluorouracil is more effective in clinical stage II (10). We hypothesized that patients with clinical stage II or III will benefit from different neoadjuvant treatment protocols and more intensive perioperative therapy is required for patients with clinical stage III. However, in stratification analysis, we found that ESCC patients with clinical stage III did not benefit from nCRT.
pCR is associated with favorable prognosis in patients with esophageal carcinoma who receive neoadjuvant treatment, and neoadjuvant CRT improves the rate of pCR compared with that of nCT (18,19). Our study showed that the pCR rate was 28.3% in the nCRT group versus 13.8% in the nCT group (P=0.06). This is different from previous studies, as higher pCR rate in nCT group was achieved by use of TP (10,19). Among patients in the nCT group of our study, none of 7 patients who undertook DF regimen achieved pCR, while 8 of 51 (15.7%) patients who undertook TP regimen showed pCR. Fan et al. reported pCR rate of 13.3% (4/35) after two cycles of TP regimen for locally advanced ESCC (20). Moreover, pCR rate reached almost 50% for AC or ESCC patients in neoadjuvant CRT with docetaxel/paclitaxel-based chemotherapy (2,21). A phase II study reported that pCR rate was 47% of combined docetaxel-based chemotherapy and radiotherapy followed by surgery for esophageal cancer, and 5-year OS for pCR, near pCR, and presence of residual tumor subsets was 77%, 44%, and 14%, respectively (P<0.001). In present study, 3-year total OS was 68.0% in pCR, but patients with pCR did not show survival advantage compared with no-pCR (21). Despite higher pCR rate in the nCRT group, this did not translate into an OS benefit. It is possible that adding a focal treatment in nCT will not have an effect on survival for patients with occult systemic metastasis.
Neoadjuvant CT may impair human immune system, and impact on wound healing and infectious morbidity. The concurrent chemoradiotherapy may lead to higher intra-postoperative incidences because of radiotherapy induced edema, inflammation and fibrosis (13). Gronnier et al. reported that postoperative anastomotic leakage rates in nCRT and surgery alone were 8.8% versus 10.6% (P=0.220), and 90-day postoperative morbidity rates were 33.4% versus 32.1% (P=0.564) (22). A randomized clinical trial also revealed no significant difference in the incidence of complications between patients in nCT and nCRT group. However, complications were significantly more severe in nCRT (23). Similarly, our study showed that total postoperative morbidity rates in nCRT group and CT group were 32.1% versus 37.9% (P=0.660), but two cases died from complications related to anastomotic leakage in nCRT group.
Our study has some limitations. This is a retrospective analysis with a small sample size. Types of surgery and chemotherapy protocols between groups were not balanced among the patients, which may impair explanatory power. In addition, the follow-up period in nCT group was too short, which would affect the accuracy of the outcome.
Conclusions
Compared with nCT, neoadjuvant CRT may improve the pCR rate in patients with locally advanced ESCC but does not increase the risk of postoperative morbidity. Neoadjuvant CRT or CT can achieve favorable results with regard to OS and DFS. A large multi-center, randomized, prospective trial is needed to prove whether CRT or CT is better.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.34). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional review board of Zhengzhou University Affiliated Cancer Hospital (2016ct127). Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33. [Crossref] [PubMed]
- Allum WH, Stenning SP, Bancewicz J, et al. Long-Term Results of a Randomized Trial of Surgery With or Without Preoperative Chemotherapy in Esophageal Cancer. J Clin Oncol 2009;27:5062-7. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [Crossref] [PubMed]
- Ruol A, Castoro C, Portale G, et al. Trends in Management and Prognosis for Esophageal Cancer SurgeryTwenty-five Years of Experience at a Single Institution. Arch Surg 2009;144:247-54. [Crossref] [PubMed]
- Talsma AK, Damhuis RA, Steyerberg EW, et al. Determinants of improved survival after oesophagectomy for cancer. Br J Surg 2015;102:668-75. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Hoeppner J, Zirlik K, Brunner T, et al. Multimodal treatment of locally advanced esophageal adenocarcinoma: which regimen should we choose? Outcome analysis of perioperative chemotherapy versus neoadjuvant chemoradiation in 105 patients. J Surg Oncol 2014;109:287-93. [Crossref] [PubMed]
- Luu TD, Gaur P, Force SD, et al. Neoadjuvant chemoradiation versus chemotherapy for patients undergoing esophagectomy for esophageal cancer. Ann Thorac Surg 2008;85:1217-23. [Crossref] [PubMed]
- Nakamura K, Kato K, Igaki H, et al. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol 2013;43:752-5. [Crossref] [PubMed]
- Polee MB, Tilanus HW, Eskens FA, et al. Phase II study of neoadjuvant chemotherapy with paclitaxel and cisplatin given every 2 weeks for patients with a resectable squamous cell carcinoma of the esophagus. Ann Oncol 2003;14:1253-7. [Crossref] [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol 2005;23:4330-7. [Crossref] [PubMed]
- Luc G, Vendrely V, Terrebonne E, et al. Neoadjuvant chemoradiotherapy improves histological results compared with perioperative chemotherapy in locally advanced esophageal adenocarcinoma. Ann Surg Oncol 2015;22:604-9. [Crossref] [PubMed]
- Fan Y, Jiang YH, Zhou XM, et al. Clinical outcomes following neoadjuvant nab-paclitaxel and cisplatin chemotherapy for locally advanced esophageal squamous cell carcinoma. J Clin Oncol 2015;suppl 33: abstr 4055.
- Pasini F, de Manzoni G, Zanoni A, et al. Neoadjuvant Therapy With Weekly Docetaxel and Cisplatin,5-Fluorouracil Continuous Infusion, and Concurrent, Radiotherapy in Patients With Locally Advanced Esophageal Cancer Produced a High Percentage of Long-Lasting, Pathological Complete Response. Cancer 2013;119:939-45. [Crossref] [PubMed]
- Gronnier C, Tréchot B, Duhamel A, et al. Impact of neoadjuvant chemoradiotherapy on postoperative outcomes after esophageal cancer resection: results of a European multicenter study. Ann Surg 2014;260:764-70. [Crossref] [PubMed]
- Klevebro F, Johnsen G, Johnson E, et al. Morbidity and mortality after surgery for cancer of the oesophagus and gastro-oesophageal junction: A randomized clinical trial of neoadjuvant chemotherapy vs. neoadjuvant chemoradiation. Eur J Surg Oncol 2015;41:920-6. [Crossref] [PubMed]


