
IL-6, cancer and cachexia: metabolic dysfunction creates the perfect storm
Interleukin-6 (IL-6) is a pleiotropic cytokine with a wide range of biological actions including immune function, metabolism, hematopoiesis, and oncogenesis (1,2). IL-6 can have both pro and anti-inflammatory functions depending on the condition. Initially, IL-6 was believed to be pro-inflammatory because several inflammatory-based disorders such as infection, diabetes and obesity commonly show an increase in circulating IL-6 (3-5). In contrast, transient expression of IL-6 after exercise can have anti-inflammatory and insulin sensitizing effects (3,6,7). Despite its enigmatic biology, IL-6 has been heavily investigated for its role in the progression of cancer and cancer-associated cachexia (8). The recent publication from Flint et al. (9) adds supporting evidence that IL-6 regulates these processes through direct and indirect targeting of multiple systems leading to alterations in metabolism and endocrine function.
IL-6 signaling
The IL-6 family signals through the membrane bound receptor gp130 (glycoprotein 130) (6). Upon binding, the gp130 receptor activates JAK (Janus Kinase) tyrosine kinase (6). Activation of JAK-1 leads to phosphorylation of tyrosines in the cytoplasmic domain of gp130, which enables binding for proteins with Src homology domain-2 (SH2) and phosphotyrosine-binding proteins (10). Among these proteins are the STATs, which are critical for IL-6 signaling (10,11). STAT proteins are activated by phosphorylation on a single tyrosine residue leading to the formation of STAT dimmers (10,11). The STAT dimmers can then translocate to the nucleus where they function with other factors to increase the transcription of genes containing STAT-responsive elements in their promoters (10,11). IL-6 can activate both STAT-1 and STAT-3, however, STAT-3 has been shown to have a more pronounced role in the IL-6 signaling pathway (10). STAT-3 transcriptional targets are involved in multiple cellular functions including immune function, cell proliferation and growth, differentiation and possibly apoptosis (12,13). The gp130 receptor is found in most tissues throughout the body enabling potent and diverse effects of IL-6 especially during heightened expression conditions including cancer and cancer-associated cachexia.
IL-6 and muscle protein turnover
Although strong evidence supports the deleterious role of IL-6 during cachexia, IL-6 treatment in non-tumor bearing rodents creates difficulty in determining a mechanism for IL-6 in muscle wasting. Some reports claim a direct role for IL-6 to regulate wasting. Transgenic mice over-expressing circulating IL-6 show muscle atrophy associated with increased expression of lysosomal and ubiquitin-related mRNA and proteins (14,15). In humans, IL-6 administration caused a reduction in skeletal muscle protein synthesis and an increased uptake by non-muscle tissues, a condition similar to what is observed in cachexia (16). In cell culture, C2C12 myotubes treated with IL-6 have increased lysosomal and ubiquitin-related proteins and enzymatic activity (17). In contrast to these findings, IL-6 administration in rats failed to activate muscle protein breakdown (18). IL-6 treatment to L6 myotubes was also unable to increase protein degradation (19). Together, it remains unclear whether IL-6 can directly alter skeletal muscle protein turnover under disease-free conditions but its potency to induce wasting is far greater in tumor bearing conditions. Thus, understanding the interaction(s) of IL-6 action in the context of cancer is certainly warranted.
IL-6 and cachexia
IL-6 has a well-established association with the onset of cachexia in both rodent and human wasting conditions (8). In the ApcMin/+ mouse, a model of colorectal cancer and IL-6-dependent cachexia, over-expression of IL-6 can accelerate the loss of muscle and fat along with increased tumor number (20). Furthermore, IL-6 levels corresponded with the extent of muscle wasting, suppression of protein synthesis and elevated protein degradation (21). Deletion of the IL-6 gene prevented the development of cachexia in ApcMin/+ mice despite the existence of intestinal polyps (20). Attempts have been made to manipulate the systemic actions of IL-6 and other pro-inflammatory cytokines during cancer cachexia. The use of IL-6 receptor antibody has shown to be effective against muscle wasting in tumor bearing mice. Inhibition of IL-6 activity through an IL-6 receptor antibody prevented muscle wasting in C-26 tumor bearing mice (22,23) yet had no effect on tumor size. However, questions still remain as to what secondary mechanisms were active/inactive during the induction and suppress of IL-6 as it related to the progression of cachexia.
IL-6 and immune function
IL-6 is associated with a pro inflammatory immune response during infection and injury. The recent report by Flint et al. (9) suggests a role of IL-6 to drive glucocorticoid secretion which, in turn suppresses tumor immunity. Although glucocorticoids are commonly used to suppress inflammation in diseases such as rheumatoid arthritis and Duchene’s muscular dystrophy, elevated glucocorticoids in the presents of cancer could suppress host immunity and slow or prevent anti-cancer defenses. In fact, the extent of immune cell infiltration, especially T cells, in cancer patients predicts survival and chemotherapy outcomes (24). Based on these findings, glucocorticoid levels should be considered and perhaps targeted in conjunction with immunotherapy interventions.
IL-6, anorexia and hypermetabolism
Regardless of physiological condition, a chronic state of hypermetabolism coupled with a propensity for caloric deficiency will promote eventual wasting. In terms of anorexia, cancer cachexia is often but not always linked with a reduction in caloric intake. Intriguingly, cachexia cannot be corrected through nutritional supplementation. The numerous mouse models of cachexia tend to vary in extent of anorexia (25). Human data appears more consistent in supporting a major role for anorexia in the loss of body mass (26,27). Although mechanisms are starting to be elucidated, pro inflammatory cytokines are still thought to be key mediators in the induction of anorexia during disease states (28,29). IL-6 has been shown to regulate food intake and metabolism (30), signaling through neural gp130 receptors (31). Even in non-cancer related cachexia, plasma IL-6 is associated with the incidence of anorexia (32).
The hypermetabolic state observed with cancer comes from several factors. One such mechanism is the increase in thermogenesis, as we know cancer increases the “browning” of white adipose tissue (26). Brown adipose cells use uncoupled respiration, burning glucose and lipid to generate heat instead of ATP. Activation of these cells increases whole body energy expenditure and are a pharmacological target for weight loss with obesity (33). However, in the context of cancer, activation of these cells could be detrimental. Although tumor-derived “browning” factors are starting to be identified (34), IL-6 is once again a driving mechanism to brown adipose tissue during cancer (26). Blocking inflammation or inhibition of β adrenergic signaling will attenuate cachexia progression (26) validating the hypermetabolic condition as a true contributor of wasting.
IL-6 drives hormonal dysfunction through metabolic alterations
Hepatic dysfunction is common across various cancers. Flint et al. (9) provides evidence for IL-6-induced liver dysfunction being a transitional stage into the onset of cachexia. In the ApcMin/+ mouse model, cachexia progression is associated with increased hepatic STAT signaling, apoptosis and ER stress (35). Administration of the anti-inflammatory compound pyrrolidine dithiocarbamate (PDTC) for just 2 weeks attenuated the loss of liver lipid and glycogen stores which was associated with the attenuation of cachexia progression (36). Flint et al. (26) linked IL-6 with the reduction in liver PPAR alpha mRNA expression and the suppression of ketogenesis during cancer-associated caloric deficiency. This leads to the production of glucocorticoids, which appears to be a key mediator in the transition into cachexia, thus linking metabolic dysfunction to hormonal alterations.
Increased glucocorticoid secretion will induce muscle atrophy through direct mechanisms including suppression of amino acid import (37), suppression of IGF-1 signaling and increasing myostatin signaling (38). Beyond the direct effects of glucocorticoids, indirect effects on hormonal regulation can contribute to muscle wasting as well. Disruptions in key anabolic hormones such as IGF-1 and testosterone have been reported during cachexia in both human (39,40) and mouse models of cachexia (41,42). The induction of glucocorticoids could be an underlying mechanism for the onset of hypogonadism as cortisol or pharmacological derivatives can directly suppress the production of testosterone (43) by inhibiting leydig cell function (44). Hypogonadism leads to muscle wasting and reduction in functional strength with (39,40) or without (45,46) an underlying disease. Several human diseases associated with a loss of lean body mass such as diabetes (47), chronic obstructive pulmonary disorder (COPD) (48), HIV-AIDs (49) and cancer (50) have reported a reduction in circulating testosterone in patients. Due to the anabolic properties of testosterone and its pharmacological derivatives, a strong research focus has been given to maintain anabolic hormones during catabolic conditions (51-53). Furthermore, testosterone and other anabolic steroids have shown to be effective in rescuing the loss of muscle mass in wasting conditions (54). In relation to IL-6, hypogonadism in healthy older men was associated with increased levels of circulating IL-6 while testosterone replacement returned IL-6 levels back to baseline (55). In the mouse, IL-6 over-expression does not directly suppress testosterone in pre cachectic mice while IL-6 inhibition through a IL-6 receptor antibody attenuated the drop in testosterone observed in cachectic mice (41). Together, glucocordicoid-induced suppression of testosterone could be another indirect mechanism promoting the induction of cachexia.
Conclusions
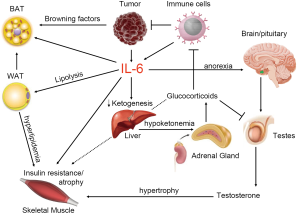
Chronic IL-6 over-expression with cancer appears to be a catalyst for multi-system dysfunction leading to accelerated tumor growth and cachexia progression (Summarized in Figure 1). The tumor and corresponding immune activation increase circulating IL-6 which starts the chain reaction. Tissues become insulin resistant caused in part by hyperlipidemia from excessive adipose lypolysis and metabolic dysfunction in skeletal muscle. This puts pressure on the liver to generate ketone bodies to provide fuel for vital tissues including the brain and heart. At the same time, IL-6 can induce a state of hypermetabolism from browning of white adipose tissue adding more pressure to ensure adequate ketone production. As the liver typically generates ketones during low energy/insulin resistance, high IL-6 levels suppress hepatic ketone production by inhibiting PPAR alpha causing a metabolic strain. As IL-6 can also promote anorexia, caloric deficiency in a background of hypermetabolism will only exacerbate the metabolic strain and cause secretion of glucocorticoids from the adrenals. The continuation of caloric deficiency and increased glucocorticoid levels accelerates the progression of cachexia through suppression of anabolic steroids, direct atrophic signaling in skeletal muscle and systemic insulin resistance. Furthermore, elevated glucocorticoids suppress immune function leaving a limited capacity for the host to slow further tumor progression. In addition, glucocorticoids will suppress commonly used anti-cancer immunotherapy. As suggested by Flint et al., IL-6 and glucocorticoid levels should be considered before initiating a treatment plan for cancer patients to maximize efficacy.
Acknowledgments
Funding: J.P.W. was supported by the NIA Pepper Center grant AG028716 and start up funds from Duke University.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Zhen-Yu Lin (Cancer center, Union hospital, Huazhong University of Science and Technology, Wuhan, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.52). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Al-Khalili L, Bouzakri K, Glund S, et al. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol 2006;20:3364-75. [Crossref] [PubMed]
- Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res 2002;4:S233-42. [Crossref] [PubMed]
- Kern PA, Ranganathan S, Li C, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 2001;280:E745-51. [PubMed]
- Pickup JC, Mattock MB, Chusney GD, et al. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997;40:1286-92. [Crossref] [PubMed]
- Eder K, Baffy N, Falus A, et al. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res 2009;58:727-36. [Crossref] [PubMed]
- Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol 2003;149:1-38. [Crossref] [PubMed]
- Pedersen BK. IL-6 signalling in exercise and disease. Biochem Soc Trans 2007;35:1295-7. [Crossref] [PubMed]
- Narsale AA, Carson JA. Role of interleukin-6 in cachexia: therapeutic implications. Curr Opin Support Palliat Care 2014;8:321-7. [Crossref] [PubMed]
- Flint TR, Janowitz T, Connell CM, et al. Tumor-Induced IL-6 Reprograms Host Metabolism to Suppress Anti-tumor Immunity. Cell Metab 2016;24:672-84. [Crossref] [PubMed]
- Croker BA, Krebs DL, Zhang JG, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol 2003;4:540-5. [Crossref] [PubMed]
- Heinrich PC, Behrmann I, Haan S, et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 2003;374:1-20. [Crossref] [PubMed]
- Levy DE, Darnell JE Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002;3:651-62. [Crossref] [PubMed]
- Clarkson RW, Boland MP, Kritikou EA, et al. The genes induced by signal transducer and activators of transcription (STAT)3 and STAT5 in mammary epithelial cells define the roles of these STATs in mammary development. Mol Endocrinol 2006;20:675-85. [Crossref] [PubMed]
- Tsujinaka T, Fujita J, Ebisui C, et al. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Invest 1996;97:244-9. [Crossref] [PubMed]
- Tsujinaka T, Ebisui C, Fujita J, et al. Muscle undergoes atrophy in association with increase of lysosomal cathepsin activity in interleukin-6 transgenic mouse. Biochem Biophys Res Commun 1995;207:168-74. [Crossref] [PubMed]
- van Hall G, Steensberg A, Fischer C, et al. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab 2008;93:2851-8. [Crossref] [PubMed]
- Ebisui C, Tsujinaka T, Morimoto T, et al. Interleukin-6 induces proteolysis by activating intracellular proteases (cathepsins B and L, proteasome) in C2C12 myotubes. Clin Sci (Lond) 1995;89:431-9. [Crossref] [PubMed]
- García-Martínez C, Lopez-Soriano FJ, Argiles JM. Interleukin-6 does not activate protein breakdown in rat skeletal muscle. Cancer Lett 1994;76:1-4. [Crossref] [PubMed]
- Williams A, Wang JJ, Wang L, et al. Sepsis in mice stimulates muscle proteolysis in the absence of IL-6. Am J Physiol 1998;275:R1983-91. [PubMed]
- Baltgalvis KA, Berger FG, Pena MM, et al. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol 2008;294:R393-401. [Crossref] [PubMed]
- White JP, Baynes JW, Welle SL, et al. The Regulation of Skeletal Muscle Protein Turnover during the Progression of Cancer Cachexia in the Apc Mouse. PLoS One 2011;6:e24650 [Crossref] [PubMed]
- Strassmann G, Fong M, Kenney JS, et al. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest 1992;89:1681-4. [Crossref] [PubMed]
- Fujita J, Tsujinaka T, Yano M, et al. Anti-interleukin-6 receptor antibody prevents muscle atrophy in colon-26 adenocarcinoma-bearing mice with modulation of lysosomal and ATP-ubiquitin-dependent proteolytic pathways. Int J Cancer 1996;68:637-43. [Crossref] [PubMed]
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. [Crossref] [PubMed]
- Bennani-Baiti N, Walsh D. Animal models of the cancer anorexia-cachexia syndrome. Support Care Cancer 2011;19:1451-63. [Crossref] [PubMed]
- Wigmore SJ. Contribution of anorexia and hypermetabolism to weight loss in anicteric patients with pancreatic cancer. Br J Surg 1997;84:196-7. [Crossref] [PubMed]
- Inui A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J Clin 2002;52:72-91. [Crossref] [PubMed]
- Teli T, Xanthaki D, Karalis KP. Regulation of appetite and insulin signaling in inflammatory states. Ann N Y Acad Sci 2006;1083:319-28. [Crossref] [PubMed]
- Seruga B, Zhang H, Bernstein LJ, et al. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer 2008;8:887-99. [Crossref] [PubMed]
- Benrick A, Schéle E, Pinnock SB, et al. Interleukin-6 gene knockout influences energy balance regulating peptides in the hypothalamic paraventricular and supraoptic nuclei. J Neuroendocrinol 2009;21:620-8. [Crossref] [PubMed]
- Plata-Salamán CR. Anorexia induced by activators of the signal transducer gp 130. Neuroreport 1996;7:841-4. [Crossref] [PubMed]
- Bossola M, Luciani G, Giungi S, et al. Anorexia, fatigue, and plasma interleukin-6 levels in chronic hemodialysis patients. Ren Fail 2010;32:1049-54. [Crossref] [PubMed]
- Cohen P, Spiegelman BM. Brown and Beige Fat: Molecular Parts of a Thermogenic Machine. Diabetes 2015;64:2346-51. [Crossref] [PubMed]
- Kir S, White JP, Kleiner S, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 2014;513:100-4. [Crossref] [PubMed]
- Narsale AA, Enos RT, Puppa MJ, et al. Liver inflammation and metabolic signaling in ApcMin/+ mice: the role of cachexia progression. PLoS One 2015;10:e0119888 [Crossref] [PubMed]
- Narsale AA, Puppa MJ, Hardee JP, et al. Short-term pyrrolidine dithiocarbamate administration attenuates cachexia-induced alterations to muscle and liver in ApcMin/+ mice. Oncotarget 2016;7:59482-502. [PubMed]
- Hasselgren PO, Fischer JE. Counter-regulatory hormones and mechanisms in amino acid metabolism with special reference to the catabolic response in skeletal muscle. Curr Opin Clin Nutr Metab Care 1999;2:9-14. [Crossref] [PubMed]
- Hanaoka BY, Peterson CA, Horbinski C, et al. Implications of glucocorticoid therapy in idiopathic inflammatory myopathies. Nat Rev Rheumatol 2012;8:448-57. [Crossref] [PubMed]
- Garcia JM, Li H, Mann D, et al. Hypogonadism in male patients with cancer. Cancer 2006;106:2583-91. [Crossref] [PubMed]
- Grinspoon S, Corcoran C, Lee K, et al. Loss of lean body and muscle mass correlates with androgen levels in hypogonadal men with acquired immunodeficiency syndrome and wasting. J Clin Endocrinol Metab 1996;81:4051-8. [PubMed]
- White JP, Puppa MJ, Narsale A, et al. Characterization of the male ApcMin/+ mouse as a hypogonadism model related to cancer cachexia. Biol Open 2013;2:1346-53. [Crossref] [PubMed]
- Costelli P, Muscaritoli M, Bossola M, et al. IGF-1 is downregulated in experimental cancer cachexia. Am J Physiol Regul Integr Comp Physiol 2006;291:R674-83. [Crossref] [PubMed]
- Cumming DC, Quigley ME, Yen SS. Acute suppression of circulating testosterone levels by cortisol in men. J Clin Endocrinol Metab 1983;57:671-3. [Crossref] [PubMed]
- Welsh TH. Jr, Bambino TH, Hsueh AJ. Mechanism of glucocorticoid-induced suppression of testicular androgen biosynthesis in vitro. Biol Reprod 1982;27:1138-46. [Crossref] [PubMed]
- Katznelson L, Finkelstein JS, Schoenfeld DA, et al. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 1996;81:4358-65. [PubMed]
- Mauras N, Hayes V, Welch S, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab 1998;83:1886-92. [PubMed]
- Dhindsa S, Prabhakar S, Sethi M, et al. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab 2004;89:5462-8. [Crossref] [PubMed]
- Van Vliet M, Spruit MA, Verleden G, et al. Hypogonadism, quadriceps weakness, and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;172:1105-11. [Crossref] [PubMed]
- Cohan GR. HIV-associated hypogonadism. AIDS Read 2006;16:341-5, 348, 352-4. [PubMed]
- Dev R, Hui D, Del Fabbro E, et al. Association between hypogonadism, symptom burden, and survival in male patients with advanced cancer. Cancer 2014;120:1586-93. [Crossref] [PubMed]
- Kadi F. Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br J Pharmacol 2008;154:522-8. [Crossref] [PubMed]
- Simon D, Charles MA, Lahlou N, et al. Androgen therapy improves insulin sensitivity and decreases leptin level in healthy adult men with low plasma total testosterone: a 3-month randomized placebo-controlled trial. Diabetes Care 2001;24:2149-51. [Crossref] [PubMed]
- Laaksonen DE, Niskanen L, Punnonen K, et al. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol 2003;149:601-8. [Crossref] [PubMed]
- Bhasin S, Storer TW. Anabolic applications of androgens for functional limitations associated with aging and chronic illness. Front Horm Res 2009;37:163-82. [Crossref] [PubMed]
- Khosla S, Atkinson EJ, Dunstan CR, et al. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. J Clin Endocrinol Metab 2002;87:1550-4. [Crossref] [PubMed]


