
NLRC3 mediated PI3K-mTOR inhibition takes a toll on colon cancer
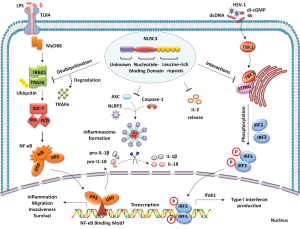
NLRs (nucleotide-binding domain and leucine-rich repeats containing receptors) are pattern recognition receptors, evolutionarily conserved across plants and animals (1). NLRs, initiate immune response against specific damage and pathogen-associated molecular patterns (DAMPs/PAMPs) (2). NLRs also contribute to the function and regulation of multiple innate immune signaling pathways (3) (Figure 1). NLRC3, the newly characterized NLR family member is preferentially expressed in immune cells. NLRC3 performs various innate immune functions such as regulation of several inflammatory signaling pathways (4). NLRC3 attenuates TLR (TOLL-like receptor) signaling via inhibition of MyD88 and TRAF6-dependent activation of NF-κB pathway. In fact, NLRC3 interacts with TRAF6 to promote its degradation (5). According to Gultekin et al., NLRC3 is expressed as a cytosolic protein and promotes negative regulation of inflammasome signaling. Inflammasome represents a multi-protein platform comprising of a NLR family member, apoptosis-associated speck-like protein containing CARD (ASC) and pro-caspase-1, a cysteine protease enzyme (2). NLRC3 specifically inhibits NLRP3-induced ASC speck formation, and subsequent activation of pro-caspase-1 and IL-1β production (6) (Figure 1). Furthermore, NLRC3 interacts with stimulator of interferon genes (STING) to modulate host response towards intracellular DNA, DNA virus, and cyclic di-GMP. NLRC3 impedes STING-TBK1 interaction, reducing STING-dependent innate immune activation and downstream type I interferon production (7). The STING pathway mediates recognition of cytosolic DNA by dendritic cells, generating spontaneous T cell responses against immunogenic tumors (8). However, study by Corrales et al., highlights another major pathway triggered by tumor-derived DNA that activates AIM2 inflammasome. The AIM2 inflammasome activation inhibits STING pathway, by promoting caspase-1 mediated cell death (9). In summary, these findings highlight the complex regulation of inflammasomes, and their association with other innate immune signaling pathways.
Dysregulation of NLR signaling is central to several inflammation-associated diseases including cancer (3). NLR signaling plays a critical role in cancer initiation, development and progression. Moreover, NLRs perform both pro- and anti-tumor regulatory roles in cancer (10). Specifically, colorectal cancer presents disease burden worldwide, and is the third leading cause of cancer death in U.S (11). The dramatic increase in colon cancer incidence and mortality rate, has raised attempts towards potential targets identification, to reduce cancer risk, promote preventive diagnosis and effective treatment strategy. Scientists have identified the novel association of NLR family members, NLRP6 and NLRP12 with tumorigenesis in colorectal cancer (12). Both NLRP6 and NLRP12 are non-inflammasome-forming NLRs and function as negative regulators of inflammation signaling. The Nlrp6−/− and Nlrp12−/− mice showed increased susceptibility towards inflammation-driven colon tumorigenesis. Importantly, Nlrp6 and Nlrp12 signaling in the hematopoietic cells is critical for protection against colitis and colitis-associated tumorigenesis (13-15). Emerging data provides strong evidence for NLRs-mediated protection in colorectal cancer, and suggest interplay between NLRs and inflammation-associated molecules during colitis and colitis-associated tumorigenesis.
Recently, Karki and Man et al., identified NLRC3 as a negative regulator of PI3K-mTOR pathway in colorectal cancer (16). NLRC3 expression was significantly higher in the colon tumor tissue as compared to the non-tumor/normal. The study utilized azoxymethane (AOM)-dextran sulfate sodium (DSS) model for colorectal tumorigenesis. The reduced body weight and colon length, and increased dysplasia and adenocarcinoma in the AOM-DSS treated Nlrc3−/− mice, signifies the protective role of NLRC3 in colitis-associated colorectal cancer. To assess the contribution of any gene-deletion induced microbiota alterations, the mice were co-housed during the course of experiments. However, results remained unchanged in the co-housed and non-co-housed experimental groups confirming the lack of microbiota contribution. Interestingly, the Nlrc3-deficient mice when treated with AOM-DSS had significantly increased weight loss, tumor size and tumor number per colon as compared to the wild type controls. Histopathological analysis further revealed higher colon inflammation, ulceration, hyperplasia and extent of severity/damage in the Nlrc3−/− mice as compared to wild-type mice (16). Based on the preliminary observations, NLRC3 emerges as a potential innate immune regulator for protection against colorectal tumorigenesis.
To elucidate cell-specific regulation of NLRC3 during colorectal tumorigenesis, Karki and Man et al. performed bone-marrow chimera experiments (16) . Nlrc3−/− mice showed significant increase in tumor size and number as compared to wild-type mice, after wild-type bone marrow transplant. Similarly, wild-type mice when transplanted with Nlrc3−/− bone marrow had significantly increased tumor size with respect to wild-type mice that received wild-type bone marrow. Notably, Nlrc3−/− mice, with Nlrc3−/− bone marrow showed highest tumor load compared to the other transplants (16). Previous studies demonstrated cell-specific role of inflammasome-forming NLRs, NLRP3 and NLRC4 in colon cancer (17,18). Both Nlrp3−/− and Nlrp4−/− mice were highly susceptible to DSS-induced colitis and suffered loss of epithelial integrity during colitis-associated tumorigenesis. The authors further confirmed these results by generating mice lacking NLRC3 in either hematopoietic or myeloid or intestinal epithelial cells. The Nlrc3−/− mice showed higher tumor development, followed by the mice lacking Nlrc3 in hematopoietic cells. Hence, the inhibitory effect of NLRC3 on tumor growth is significant in epithelial cells as compared to the hematopoietic cells.
In their work, Karki and Man et al. investigated the effect of NLRC3 on cell growth and proliferation in the AOM-DSS treated mice. Interestingly, Nlrc3−/− mice showed increased cellular proliferation in intestinal epithelial cells, which was further confirmed by increased organoids formation by the colonic epithelial stem cells and primary fibroblasts (16). The mammalian target of rapamycin (mTOR), acts downstream of phosphoinositide 3-kinase (PI3K) /Akt pathway, and its activation has been implicated in cell metabolism, proliferation, growth, migration including colon tumorigenesis (19,20). Therefore, Karki and Man et al. compared the phosphorylation levels of mTOR and mTOR-associated signaling proteins in the colon tissue of AOM-DSS treated mice. As expected, the colon of Nlrc3−/− mice at day-4 had increased phosphorylation levels of S6 kinase, 4E-BP1 and AKT at Ser473, the downstream targets of mTOR, as compared to the wild-type mice. Immunoblotting and immunofluorescence analysis showed high levels of phosphorylated AKT, total AKT, phosphorylated 4EBP1 and GAPDH in the colon tissue of Wild type, Nlrc3+/− and Nlrc3−/− mice (day-14) and in the IGF-1-treated primary fibroblasts (16). The data shows early dysregulation of mTOR in the Nlrc3−/− mice, that occurs just 8 days after injection of azoxymethane. These preliminary findings implicate NLRC3 as potent regulator of the mTOR signaling pathway.
The overactivation of Wnt-signaling promotes colorectal cancer invasion, metastasis and metabolism (21). However, Karki and Man et al. didn’t find any difference in expression of genes involved in Wnt-signaling pathways. No significant difference in the production of inflammatory mediators and immune cell infiltration was observed in the Nlrc3−/− mice at day-8. The data clearly indicates absence of NF-κB activation at early stage of tumorigenesis. Surprisingly, the pro-inflammatory mediators, IL-1β, IL-6, TNF-α, GCSF, KC, MCP-1 and MIP-1α in the colon tissue, had significantly increased relative gene and protein expression levels in treated Nlrc3−/− mice at day-14 (16). Therefore, the dysregulation of mTOR signaling was followed by NF-κB signaling activation at a later stage in tumorigenesis. Additionally, the expression of IL-17 and IL-22 was elevated in the colon tissue of Nlrc3−/− mice whereas the expression of IL-23, IFNβ and IFNγ remained unchanged at day-14. As expected, the phosphorylation levels of Iκ-Bα and STAT3 were found to be increased in the Nlrc3−/− mice as compared to the wild-type mice at day-14. Consistent with the observations that Nlrc3−/− mice had increased expression of pro-inflammatory mediators, the authors observed significantly higher number of macrophages, CD11b+ CD11c+ cells, neutrophils, and NK cells, in the colon tissue of Nlrc3−/− mice at day-14 (16). The results demonstrate NLRC3-controlled reduction in intestinal inflammation and immune cell infiltration during colorectal tumorigenesis. Several upstream signaling molecules regulate the phosphorylation and activation of mTOR in the PI3K–AKT–mTOR pathway (22). The phosphorylation of AKT at the Thr308 site by the kinase PDK1 activates the mTOR regulated pathways (23). The authors found increased phosphorylation of AKT at Thr308 site in the Nlrc3−/−, followed by the heterozygous Nlrc3+/− with respect to wild-type. Therefore, NLRC3 suppresses colon tumorigenesis in gene-dose dependent manner, via PI3K-mTOR-AKT pathway response.
The authors further evaluated NLRC3-mediated protection mechanism against colorectal cancer, using the Apcmin/+ mice, a spontaneous model of colon cancer. They found increased cellular proliferation, dysplasia and tumor burden in the colon of Apcmin/+Nlrc3−/− mice. Moreover, the colon of Apcmin/+Nlrc3−/− mice, showed decreased proliferation, inflammation damage and tumor load in the presence of mTOR/PI3K/mTOR-PI3K inhibitors (16). All above investigations present NLRC3 as a negative regulator of mTOR to prevent colorectal cancer progression. NLRC3 interactions with the PDK1 and p85 subunit of PI3K were confirmed by co-immunoprecipitation assays, in the IGF-1 activated Nlrc3−/− primary fibroblasts/bone-marrow-derived macrophages (BMDMs). Increased phosphorylation and activation of p85 subunit was observed in the colon tissue of AOM-DSS treated Nlrc3−/− mice as compared to the wild-type. Collectively, the findings demonstrate NLRC3-mediated disruption of p85-p110α PI3K subunits interactions, by direct interaction with the p85 subunit. Researchers also found increased activation of mTOR signaling, in response to LPS-induced TLR4 activation, in the Nlrc3−/− primary BMDMs and Nlrc3ld/ld mice (ld- large deletion) (16). Here, the Nlrc3ld/ld mice represent the independently generated NLRC3-deficient mice in the laboratory.
Recent findings by Karki and Man et al., highlights the protective role of NLRC3 against colorectal cancer, via inhibition of the PI3K-mTOR-AKT signaling pathway. The results explain the molecular mechanism of NLRC3 mediated inhibition of cellular proliferation, inflammation and immune cell infiltration in colon cancer (16). The emerging evidences suggest cross-talk between NLRC3 and major intracellular signaling pathways, involved in cell growth, proliferation and inflammation. NLRC3 is a negative regulator of inflammation and it would be interesting to find the association of NLRC3 with inflammasome-forming NLRs, during colorectal tumorigenesis. Several new questions arise from these findings: (I) How is NLRC3 expression in epithelial cells but not hematopoietic cells critical to tumorigenesis? (II) How does NLRC3-mediated signaling vary across cell lineages? (III) Is NLRC3 expression variable or is the difference in signaling arising from NLRC3-interacting signaling proteins? From the preferential expression of NLRC3 in innate immune cells, it becomes important to further understand the structural and activation mechanism of NLRC3 and its downstream target molecules. These questions and many more arising from this seminal work will fuel future research in comprehending the cellular and molecular pathways underlying NLRC3-mediated protection against colorectal cancer.
Inflammation and innate immune cell infiltration are major components of the tumor microenvironment. The significance of NLRs in generation of host innate immune responses has led to the development of NLR-targeted drugs against inflammation-associated diseases and cancer (24,25). The newly identified role of NLRC3 in protection against colorectal cancer has generated a lot of curiosity regarding the expression and function of NLRC3 in other cancers. NLRC3, similar to its other family members, presents a promising option for development of NLR-targeted strategies, to foster host immune responses against the tumor-microenvironment.
Acknowledgments
The software application Science Slides (VisiScience) was used to generate parts of Figure 1.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Fengbo Tan, MD (Department of Gastrointestinal Surgery, Xiangya Hospital, Central South University, Changsha, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.54). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maekawa T, Kufer TA, Schulze-Lefert P. NLR functions in plant and animal immune systems: so far and yet so close. Nat Immunol 2011;12:817-26. [Crossref] [PubMed]
- Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell 2006;126:659-62. [Crossref] [PubMed]
- Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 2011;29:707-35. [Crossref] [PubMed]
- Conti BJ, Davis BK, Zhang J, et al. CATERPILLER 16.2 (CLR16. 2), a novel NBD/LRR family member that negatively regulates T cell function. Journal of Biological Chemistry 2005;280:18375-85. [Crossref] [PubMed]
- Schneider M, Zimmermann AG, Roberts RA, et al. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-κB. Nat Immunol 2012;13:823-31. [Crossref] [PubMed]
- Gültekin Y, Eren E, Özören N. Overexpressed NLRC3 acts as an anti-inflammatory cytosolic protein. J Innate Immun 2015;7:25-36. [Crossref] [PubMed]
- Zhang L, Mo J, Swanson KV, et al. NLRC3, a member of the NLR family of proteins, is a negative regulator of innate immune signaling induced by the DNA sensor STING. Immunity 2014;40:329-41. [Crossref] [PubMed]
- Woo SR, Fuertes MB, Corrales L, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014;41:830-42. [Crossref] [PubMed]
- Corrales L, Woo SR, Williams JB, et al. Antagonism of the STING Pathway via Activation of the AIM2 Inflammasome by Intracellular DNA. J Immunol 2016;196:3191-8. [Crossref] [PubMed]
- Janowski AM, Kolb R, Zhang W, et al. Beneficial and Detrimental Roles of NLRs in Carcinogenesis. Front Immunol 2013;4:370. [Crossref] [PubMed]
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [Crossref] [PubMed]
- Elinav E, Strowig T, Kau AL, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011;145:745-57. [Crossref] [PubMed]
- Allen IC, Wilson JE, Schneider M, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity 2012;36:742-54. [Crossref] [PubMed]
- Zaki MH, Vogel P, Malireddi RK, et al. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell 2011;20:649-60. [Crossref] [PubMed]
- Chen GY, Liu M, Wang F, et al. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J Immunol 2011;186:7187-94. [Crossref] [PubMed]
- Karki R, Man SM, Malireddi RK, et al. NLRC3 is an inhibitory sensor of PI3K-mTOR pathways in cancer. Nature 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Allen IC, TeKippe EM, Woodford RM, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med 2010;207:1045-56. [Crossref] [PubMed]
- Hu B, Elinav E, Huber S, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci U S A 2010;107:21635-40. [Crossref] [PubMed]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 2006;441:424-30. [Crossref] [PubMed]
- Gulhati P, Cai Q, Li J, et al. Targeted inhibition of mammalian target of rapamycin signaling inhibits tumorigenesis of colorectal cancer. Clin Cancer Res 2009;15:7207-16. [Crossref] [PubMed]
- Sebio A, Kahn M, Lenz HJ. The potential of targeting Wnt/β-catenin in colon cancer. Expert Opin Ther Targets 2014;18:611-5. [Crossref] [PubMed]
- Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol 2014;15:155-62. [Crossref] [PubMed]
- Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005;307:1098-101. [Crossref] [PubMed]
- López-Castejón G, Pelegrín P. Current status of inflammasome blockers as anti-inflammatory drugs. Expert Opin Investig Drugs 2012;21:995-1007. [Crossref] [PubMed]
- Krysko DV, Garg AD, Kaczmarek A, et al. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012;12:860-75. [Crossref] [PubMed]


