
Association between breast cancer and thyroid cancer: a descriptive study
Introduction
Thyroid cancer is one of the most common malignant tumors worldwide. In 2012, approximately 119,000 patients were diagnosed with thyroid cancer in China, among which 90,000 patients (76%) were women (1). Therefore, thyroid cancer has become the seventh most common cancer type identified in Chinese women (2). A similar epidemiology has been observed in the United States, where thyroid cancer is the fifth most prevalent cancer, accounting for 6% of new cancer diagnoses in women (3). However, although thyroid cancer has a high incidence rate, it does have a low mortality rate, and the overall 5-year survival rate of thyroid cancer patients approximates 98%. Therefore, the number of malignant disease survivors is increasing rapidly. In addition, breast cancer is the most frequently diagnosed malignancy in women worldwide, with the incidence in China of 41.32 per 100,000 women and approximately 272,700 female breast cancer patients in 2012 (1). It has been reported that thyroid cancer survivors have a greater risk of developing breast cancer compared with the general population (4-6). The relationship between the two diseases might be related to treatment of the primary malignancy, specifically radiation therapy, or it could also be due to genetic and hormonal factors (7,8). However, the exact relationship between the two cancer types is very controversial. This paper attempts to examine the frequency of co-existing breast and thyroid cancer while discussing the clinicopathological features of patients and the putative mechanism of association.
Methods
Patients
We retrospectively analyzed the medical records of thyroid cancer patients diagnosed from January 2012 to May 2016 in the General Hospital of Jinan Military Command (Jinan, China). A total of 486 female patients with thyroid cancer (papillary, follicular, medullary or anaplastic carcinoma) who underwent thyroidectomy with local or modified radical lymphadenectomy were included. Of these, eight patients suffered from breast malignancy either synchronously or metachronously. This study received approval from the Ethics Committee of the General Hospital of Jinan Military Command (No. 2013ZD01). Written informed consent was obtained from all patients.
Clinicopathological information
Characteristics and clinical information for the 486 patients with thyroid cancer are presented in Table 1. Ages ranged from 19 to 78 years, and the median age was 48 years. In total, 89.1% of thyroid tumors were papillary thyroid carcinoma (PTC). The diameter of most tumors was less than 2 cm. Local lymph node metastasis occurred in 37.0% of patients. TNM stage was utilized by the TNM-union for International Cancer Control, 2002. The 34.8% (169 cases) of patients were categorized as stage I, and 26.5%, 21.2%, and 17.5% of patients were categorized as stage II, III, and IV, respectively. 53.7% of patients received adjuvant radioactive iodine therapy.
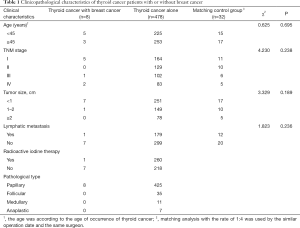
Full table
Immunohistochemistry analysis
The hormone receptors [estrogen receptor (ER) and progesterone receptor (PR)] and human epidermal growth factor receptor 2 (HER-2) status of invasive breast carcinoma were detected by immunohistochemical method using the Ventana BenchMark XT system (Roche Ltd, Basel, Switzerland). The expression status was scored as previously described (9,10). In addition, MSH2, MSH6, MLH1, and PMS2 expression were assessed by immunohistochemistry in both breast cancers and thyroid cancers. All antibodies were purchased from Roche Ltd.
Statistical analysis
SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Considering the effect of different operative approaches, matching analysis with the rate of 1:4 was used by the similar operation date and the same surgeon. Chi square and Fisher’s exact tests were carried out to compare the clinicopathological characteristics of patients with two co-existing cancers and patients with thyroid cancer alone. A value of P<0.05 was considered as a statistically significant difference.
Results
Clinicopathological characteristics
Among 486 thyroid cancer patients, eight patients suffered from breast malignancies synchronously or metachronously (Table 2). The thyroid diseases were diagnosed as PTC in all 8 patients; breast malignancies involved invasive ductal carcinoma, invasive lobular carcinoma, intraductal carcinoma in situ and Paget’s disease. Of these 8 patients suffering from primary thyroid cancer, the ages ranged from 33 to 50 years, and the median age was 45.5 years. The diameter of most (87.5%) thyroid tumors was less than 1 cm, while that was 52.5% in patients with thyroid cancer alone. However, this was not statistically different (P=0.189). Lymphatic metastasis from thyroid cancer was observed in only one (12.5%) patient in the group where two cancers were present, while it was 37.4% in patients had thyroid cancer alone (P=0.236). Moreover, five patients were stage I by the thyroid cancer TNM staging system, one patient was stage III, and two were stage IV. These patients were not significantly different from the group where thyroid cancer alone had been diagnosed (P=0.238). For the treatment of thyroid cancer, only one patient with two co-existing cancers received radioactive iodine treatment (case 3), while the number in the control group to receive such treatment was 260 of 478 cases. Six patients with invasive breast carcinoma received 6 cycles of chemotherapy, of which one patient (case 5) receiving radiotherapy at the same time as surgery and chemotherapy. One patient (case 2) with intraductal carcinoma in situ underwent 2 cycles of neoadjuvant chemotherapy before mastectomy.

Full table
In our study, 4 patients (including case 8) experienced two types of cancer within 6 months, which could be considered as synchronous diseases. Of these, 2 cases (case 6 and case 7) were admitted for masses in the breast and thyroid that were found simultaneously, and thyroid nodules were detected in one patient during follow-up for breast cancer. Although case 8 had left breast lesions excised after 3 years of subtotal thyroidectomy, the signs and symptom of Paget’s disease appeared three months after PTC. Four cases experienced subsequent PTC more than 6 months after invasive breast cancer or ductal carcinoma in situ (DCIS).
Case 1 underwent wide resection of breast cancer lesion and sentinel lymph nodes excision biopsy. In addition, 6 cycles of adjuvant chemotherapy with docetaxel (120 mg) and cyclophosphamide (0.8 g) was used. The patient had also been taking tamoxifen for a long period. Five years later, a thyroid nodule in the left lobe was found with a diameter of 0.6 cm. Total thyroidectomy and left cervical regional lymph node dissection was performed. Papillary thyroid microcarcinoma (PTMC) with metastasis to the left tracheoesophageal groove lymph nodes and thymus tissue infiltration were confirmed. Case 2 was admitted because of a right breast mass discovered 4 years previously. Needle core biopsy showed high-grade intraductal carcinoma. Two cycles of neoadjuvant chemotherapy were immediately accepted by the patient. Next, regional resection of the right breast lesion was performed while sentinel lymph nodes were excised. After 3.5 years, the patient was admitted for thyroid nodules. Postoperative pathology revealed PTMC with a diameter of 0.3 cm but was negative for lymph node metastasis. Case 3 received right modified radical mastectomy and 6 cycles of adjuvant chemotherapy (docetaxel and cyclophosphamide) due to right breast infiltrating ductal carcinoma with a diameter of 2.5 cm. Nine months after surgery, i.e., five months after chemotherapy completion, the patient underwent total thyroidectomy and dissection of the left cervical and central regional lymph nodes for PTMC with a diameter of 0.8 cm. The case also had metastasis to the local lymph nodes (8/30). Subsequently, two cycles of iodine therapy were performed. Resection of the right breast and dissection of the right axillary lymph nodes was performed in case 4. A diagnosis of infiltrating ductal carcinoma with a diameter of 1.8 cm was confirmed. Subsequently, 6 cycles of chemotherapy were used with docetaxel and cyclophosphamide. During the second chemotherapy session, i.e., one month after surgery, the patient could occasionally feel a right cervical lump with a diameter of 1.5 cm. Subsequently, the mass increased gradually. After 6 months, the patient received surgical treatment with subtotal thyroidectomy and dissection of the right cervical regional lymph nodes. Pathology showed PTMC (a diameter of 0.8 cm) and follicular thyroid adenoma (a diameter of 3.0 cm) without lymph node metastasis. We postulate that the mass found during chemotherapy might have been a thyroid adenoma.
Owing to invasive breast carcinoma identified by Mammotome excision biopsy, extensive resection of the left breast lesion and sentinel lymph nodes biopsy were conducted in case 5. During surgery, radiotherapy using 20 Gy for 45 minutes was performed. Then, 6 cycles of chemotherapy with pharmorubicin, cyclophosphamide, and capecitabine were employed. Meanwhile, local radiotherapy of the left breast (200 cGy ×20) was performed after the fourth chemotherapy session. Unfortunately, 5 months after surgery i.e., less than a month after the completion of chemotherapy, thyroid nodule lesion was found. This was confirmed as PTMC with a diameter of 0.5 cm by postoperative histopathology. For case 6, although the interval time between breast cancer surgery and thyroid cancer surgery exceeded 8 months, the patient was admitted after 2 years for thyroid nodule lesions with a right breast mass detected within one week. Color Doppler ultrasound examination revealed that both lesions were considered as malignancies. Considering that breast cancer has a higher malignancy rate, modified radical mastectomy of the right breast cancer was conducted as a first step. Infiltrating ductal carcinoma with a diameter of 5 cm and negative lymph node metastasis was confirmed by postoperative pathology. Then, EC-T (epirubicin, cytoxan and taxol) chemotherapy regimens were adopted. Three months after completion of chemotherapy, the patient underwent total thyroidectomy and dissection of bilateral cervical regional lymph nodes. PTCs with a diameter of 1.0 cm were found in the bilateral thyroid lobes, respectively. Case 7 complained of thyroid nodules and a breast mass that were found simultaneously. As ultrasound examination results revealed multiple thyroid nodules with enlarged cervical lymph nodes, total thyroidectomy and dissection of the bilateral cervical regional lymph nodes were the first treatment regimens. Ten days later, the patient underwent modified radical mastectomy. A diagnosis of invasive ductal and lobular carcinoma was confirmed. Subsequently, 6 cycles of chemotherapy with docetaxel and cyclophosphamide were conducted.
Case 8 underwent subtotal thyroidectomy and dissection of the right cervical lymph nodes because of PTC of the right thyroid lobe. As the patient was negative for lymph node metastasis, she did not receive any treatment except for oral euthyrox. Three months later, the skin of her left nipple was both red and swollen, apparently eroded, with eczema-like changes and purulent exudate. In the later 2.8 years, the patient had not received any treatment. Finally, postoperative pathology demonstrated Paget’s disease of the left nipple and DCIS of the breast tissue under the nipple. Meanwhile, relapsing PTMC was also confirmed in the left remnant thyroid.
Immunohistochemical results
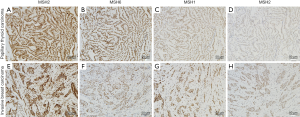
The expression states of ER, PR and HER-2 were detected in invasive breast cancer (Table 2). ER expression of 3 cases showed 3+ and the others were 2+. PR expression showed the largest variability. HER-2 expression was negative for all cases. Although case 3 was considered 2+ for immunohistochemical staining of HER-2, negative amplification was confirmed by the fluorescence in situ hybridization test. In both breast cancer and thyroid tumor samples, MSH2, MSH6, MLH1, and PMS2 were positive for all patients as detected by immunohistochemistry (Figure 1).
Discussion
In recent years, there have been many studies examining the relationship between co-existing breast cancer and thyroid disorder. Although the exact mechanism of the correlation remains unclear, many studies have shown that the incidence of concomitancy in breast cancer or thyroid cancer patients is increased compared to the general population (11,12). Consorti et al. revealed that secondary breast cancer was significantly increased in patients with thyroid cancer (13,14). Similarly, increased incidence of secondary thyroid cancer was also demonstrated in patients with breast cancer (15-18). In our retrospective study, there were 8 patients with coexisting breast cancer in 486 thyroid cancer survivors, with an incidence rate of 1.65%, which is significantly higher than the breast cancer incidence rate of 0.27% in Chinese women (2). However, we only evaluated patients with known breast disease, so there might be an element of selection bias in our study. Furthermore, considering the limited follow-up period, we believe that the incidence of breast cancer would increase in thyroid cancer patients that had an extended follow-up period. More frequent and diligent medical examination may result in the discovery of a second tumor. Furthermore, the increased incidence and excellent prognosis for longevity after identification and treatment of the primary thyroid tumor may also lead to the development of subsequent malignancies (6). Therefore, close monitoring of these patients for the detection of secondary tumor development might be beneficial in patients with thyroid cancer or breast cancer.
The role of clinicopathological characteristics in the increased incidence of this co-occurrence remains controversial. Five cases of thyroid cancer were represented as the secondary tumor but there was no significant age difference between the group with two types of cancer and the group of thyroid cancer alone. Although thyroid cancers in patients with breast cancer were represented with small size, less lymph node metastasis and occurrence in the early clinical stages, statistical difference was not observed between the two groups.
The contribution of chemotherapy or radiotherapy to the development of secondary carcinoma remains very controversial. It has been reported that positron emission tomography or chest computed tomography, which are both frequently recommended during breast cancer follow-up, may contribute to the increased rate of thyroid cancer diagnosis (5). However, Sun et al. suggest that radiotherapy does not increase the thyroid cancer risk among women with breast cancer (18). In this study, 5 cases of thyroid cancer presented during the chemotherapy or follow-up process, of which one case received radiotherapy simultaneously. The interval time between the two types of cancers, as a criterion for the judgment of secondary tumors, remains contentious. An et al. suggested that a second primary cancer was defined as that which is diagnosed at least two years after the first primary cancer (5). However, the criteria of Warren and Gates tend to be more accepted by global experts (19,20). Synchronous cancer has been defined as a tumor diagnosed simultaneously with primary cancer or within a interval time of 6 months (19). Metachronous cancer is considered as a tumor detected more than 6 months before or after diagnosis of primary cancer. Based on the latter definition, 4 cases were classified as synchronous cancers, and the other half of tumors identified in this study was consistent with metachronous cancers. Therefore, chemotherapy with docetaxel and cyclophosphamide might promote the development of thyroid cancer, but larger studies with these patients are needed to accurately determine this assumption. In the only case that received radiotherapy, thyroid cancer occurred 5 months after the first session of radiotherapy, therefore, the effect of radiotherapy on the occurrence of thyroid carcinoma could not be accurately assessed (21). However, basing on our study patients, we confirmed that the occurrence of some thyroid cancers was not related to therapy owing to the development of simultaneous breast and thyroid cancers.
In accordance with the previous studies (5), we found that all breast cancer samples were ER-positive. However, the proportion of ER expression in general breast cancer is 50–60%. Breast cancer patients with concomitant thyroid cancer had a higher expression of ER, suggesting a possible association of the molecular pathogenesis between breast cancer and thyroid cancer. It has been shown that ER levels are significantly higher in thyroid cancer compared with normal thyroid tissue (22), and estrogen can up-regulate the expression of cell cycle-related genes and proto-oncogenes in thyroid cells (7). Interestingly, all samples of invasive breast cancer were negative for HER-2 in the present study. This is consistent with a previous research (23). Chemotherapy in HER-2-negative breast cancer patients may induce cellular damage in the thyroid gland and significantly increase thyroxine-stimulating hormone (TSH) levels. Both of these factors might prompt thyroid carcinoma (23). Furthermore, Gao et al. reported three cases of HER-2-positive breast carcinoma accompanied with thyroid carcinoma in a series of 276 patients with HER-2-positive breast cancer (24).
Lynch syndrome, which is characterized by germline mutations of human mismatch repair genes, can cause an increased risk of malignancies, including colorectal cancer, endometrial carcinoma, ovarian cancer, uterine cancer, gastric cancer, and small intestine cancer (25). Recent studies reported that breast cancer and thyroid cancer also have mutations in mismatch repair genes, so they have been regarded as an expanding tumor spectrum of Lynch syndrome (26-28). Patients with Lynch syndrome are at risk to develop synchronous or metachronous tumors. MSH2, MSH6, MLH1, and PMS2 are instrumental genes of the human mismatch repair gene families, and play an important role in maintaining the integrity and stability of genetic information while avoiding genetic mutation. The normal function of the mismatch repair proteins is to proofread the nucleotide sequence for potential base-base errors that occur during DNA synthesis. Without normal DNA repair proteins, errors in replication of DNA sequences are not corrected after each replicative cycle, and mistakes made while copying the DNA template are perpetuated (29). Defects in these genes predispose to the acquisition of mutations in oncogenes and tumor-suppressor genes that play direct roles in the process of oncogenesis (29). Several studies demonstrated that women carrying MLH1 or MSH2 mutations were at increased risk of breast cancer (30-32). Harkness et al. revealed a significant threefold relative risk of breast cancer in MLH1 mutation carriers (26). Mutations of mismatch repair gene occurring in thyroid carcinoma patients with Lynch syndrome predominated in MSH2 and MLH1 genes (27). Immunohistochemistry is a sensitive and specific method used to identify defective DNA mismatch repair gene (33), so we analyzed the expression of MSH2, MSH6, MLH1, and PMS2 in breast and thyroid cancer tissues of patients with two concomitant cancers by immunohistochemical approach. Loss of PMS2 or MSH6 protein expression only, implicates mutations within the PMS2 or MSH6 genes, respectively; loss of MLH1 and PMS2 proteins suggests a MLH1 mutation; and loss of both MSH2 and MSH6 staining points to MSH2 mutations or germline deletions in the neighboring EPCAM gene. However, all mismatch repair proteins were positively expressed in our study, exhibiting no signs of Lynch syndrome in all 8 patients. Therefore, the tumorigenesis of the two cancers was not related to mutations of the human mismatch repair genes in our study.
In conclusion, there is an increased incidence of thyroid cancer and breast cancer concomitancy in patients with breast cancer or thyroid cancer. Increased surveillance of primary cancer patients may be an integral reason for the discovery of a second tumor and close monitoring for the detection of thyroid cancer or breast cancer might be beneficial for these patients. Although the underlying mechanisms remain to be elucidated, ER seems to be involved in this relationship, while DNA mismatch repair is not. More clinical studies are necessary to confirm these findings and to explore the possible mechanism behind co-occurrence of breast cancer and thyroid cancer.
Acknowledgments
The authors would like to acknowledge Elixigen Co. for the professional language editing service.
Funding: This work is supported by the President Fund of the General Hospital of Jinan Military Command (No. 2013ZD01).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.44). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of the General Hospital of Jinan Military Command (No. 2013ZD01) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res 2016;28:1-11. [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Kuo JH, Chabot JA, Lee JA. Breast cancer in thyroid cancer survivors: An analysis of the Surveillance, Epidemiology, and End Results-9 database. Surgery 2016;159:23-9. [Crossref] [PubMed]
- An JH, Hwangbo Y, Ahn HY, et al. A Possible Association Between Thyroid Cancer and Breast Cancer. Thyroid 2015;25:1330-8. [Crossref] [PubMed]
- Khang AR, Cho SW, Choi HS, et al. The risk of second primary malignancy is increased in differentiated thyroid cancer patients with a cumulative (131)I dose over 37 GBq. Clin Endocrinol (Oxf) 2015;83:117-23. [Crossref] [PubMed]
- Kumar A, Klinge CM, Goldstein RE. Estradiol-induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors alpha and beta. Int J Oncol 2010;36:1067-80. [PubMed]
- de Vathaire F, Hardiman C, Shamsaldin A, et al. Thyroid carcinomas after irradiation for a first cancer during childhood. Arch Intern Med 1999;159:2713-9. [Crossref] [PubMed]
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med 2014;138:241-56. [Crossref] [PubMed]
- Hammond ME, Hayes DF, Wolff AC. Clinical Notice for American Society of Clinical Oncology-College of American Pathologists guideline recommendations on ER/PgR and HER2 testing in breast cancer. J Clin Oncol 2011;29:e458 [Crossref] [PubMed]
- Oeffinger KC, Baxi SS, Novetsky Friedman D, et al. Solid tumor second primary neoplasms: who is at risk, what can we do? Semin Oncol 2013;40:676-89. [Crossref] [PubMed]
- Ronckers CM, McCarron P, Ron E. Thyroid cancer and multiple primary tumors in the SEER cancer registries. Int J Cancer 2005;117:281-8. [Crossref] [PubMed]
- Consorti F, Di Tanna G, Milazzo F, et al. Nulliparity enhances the risk of second primary malignancy of the breast in a cohort of women treated for thyroid cancer. World J Surg Oncol 2011;9:88. [Crossref] [PubMed]
- Subramanian S, Goldstein DP, Parlea L, et al. Second primary malignancy risk in thyroid cancer survivors: a systematic review and meta-analysis. Thyroid 2007;17:1277-88. [Crossref] [PubMed]
- Tanaka H, Tsukuma H, Koyama H, et al. Second primary cancers following breast cancer in the Japanese female population. Jpn J Cancer Res 2001;92:1-8. [Crossref] [PubMed]
- Evans HS, Lewis CM, Robinson D, et al. Incidence of multiple primary cancers in a cohort of women diagnosed with breast cancer in southeast England. Br J Cancer 2001;84:435-40. [Crossref] [PubMed]
- Vural O, Dizdar O, Petekkaya I, et al. Frequency of thyroid disease among breast cancer patients: a descriptive study of breast cancer patients. J Buon 2013;18:294-5. [PubMed]
- Sun LM, Lin CL, Liang JA, et al. Radiotherapy did not increase thyroid cancer risk among women with breast cancer: A nationwide population-based cohort study. Int J Cancer 2015;137:2896-903. [Crossref] [PubMed]
- Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and a statistical study. Am J Cancer 1932;16:1358-414.
- Lee J, Park S, Kim S, et al. Characteristics and Survival of Breast Cancer Patients with Multiple Synchronous or Metachronous Primary Cancers. Yonsei Med J 2015;56:1213-20. [Crossref] [PubMed]
- Adjadj E, Rubino C, Shamsaldim A, et al. The risk of multiple primary breast and thyroid carcinomas. Cancer 2003;98:1309-17. [Crossref] [PubMed]
- Manole D, Schildknecht B, Gosnell B, et al. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J Clin Endocrinol Metab 2001;86:1072-7. [PubMed]
- Khan MA, Bhurani D, Agarwal NB. Alteration of Thyroid Function in Indian HER 2-Negative Breast Cancer Patients Undergoing Chemotherapy. Asian Pac J Cancer Prev 2015;16:7701-5. [Crossref] [PubMed]
- Gao Q, Zheng Y, Wang B, et al. Three Metachronous Cases of HER2-Positive Breast Cancer Accompanied with Thyroid Cancer. Breast Care (Basel) 2014;9:360-3. [Crossref] [PubMed]
- Burn J, Mathers J, Bishop DT. Lynch syndrome: history, causes, diagnosis, treatment and prevention (CAPP2 trial). Dig Dis 2012;30:39-47. [Crossref] [PubMed]
- Harkness EF, Barrow E, Newton K, et al. Lynch syndrome caused by MLH1 mutations is associated with an increased risk of breast cancer: a cohort study. J Med Genet 2015;52:553-6. [Crossref] [PubMed]
- Pelizzo MR, Pennelli G, Zane M, et al. Papillary thyroid carcinoma (PTC) in Lynch syndrome: Report of two cases and discussion on Lynch syndrome behaviour and genetics. Biomed Pharmacother 2015;74:9-16. [Crossref] [PubMed]
- Ford JM. Is breast cancer a part of Lynch syndrome? Breast Cancer Res 2012;14:110. [Crossref] [PubMed]
- Clay MR, Allison KH, Folkins AK, et al. Risk of secondary malignancy (including breast) in patients with mismatch-repair protein deficiency. Am J Surg Pathol 2014;38:1494-500. [Crossref] [PubMed]
- Win AK, Lindor NM, Winship I, et al. Risks of colorectal and other cancers after endometrial cancer for women with Lynch syndrome. J Natl Cancer Inst 2013;105:274-9. [Crossref] [PubMed]
- Jensen UB, Sunde L, Timshel S, et al. Mismatch repair defective breast cancer in the hereditary nonpolyposis colorectal cancer syndrome. Breast Cancer Res Treat 2010;120:777-82. [Crossref] [PubMed]
- Walsh MD, Buchanan DD, Cummings MC, et al. Lynch syndrome-associated breast cancers: clinicopathologic characteristics of a case series from the colon cancer family registry. Clin Cancer Res 2010;16:2214-24. [Crossref] [PubMed]
- Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol 2002;20:1043-8. [Crossref] [PubMed]


