
Increased lipid desaturation and ovarian cancer stem cells
Epithelial ovarian cancer is still the most lethal gynecological malignancy (1-3) with an overall 5-year survival rate of <50% (4). This is particularly troubling considering the fact that preclinical research over the last couple of years led to significant advances in our general understanding of cancer biology, which spurred the development of numerous novel anti-cancer drugs. Unfortunately, due to the lack of specific symptoms the majority of patients (58%) are not diagnosed until the disease is already in advanced stages (III or IV) where successful treatment is difficult to achieve. Following initial, therapy-induced, remission, most patients become treatment-refractory and relapse. Recent data suggest that chemoresistance of ovarian cancer cells may be associated with a distinct cancer stem cell (CSC) phenotype (5,6). According to the CSC hypothesis, malignant tumors, just like their tissues of origin, contain a small fraction of cells that have stem-like features. These CSCs are also considered to derive from their normal counterparts, the normal tissue stem cells (5-8). These cells are characterized by their potential to self-renew and display drug resistance and low proliferative activity (5-8). Although the core of this model would provide a simple explanation for the observed biological behavior of the disease, direct identification and characterization of this particular ovarian cancer cell fraction proved to be far more complex than initially thought and is still controversially discussed (6). Specifically, the literature describing ovarian cancer cell phenotypes with stem-like properties reports a number of conflicting results (6). These discrepancies strongly argue for the necessity of a careful re-assessment of the parameters used for identification of ovarian CSC and to search for additional markers. Reliable phenotyping of ovarian CSCs is of significant clinical relevance considering that identification of the essential vulnerabilities of CSCs would enable targeting approaches for specific eradication of this crucial cancer cell population, with the realistic associated aim to develop better, curative anti-cancer therapies (7).
Interestingly, recent data indicate that microenvironmental factors (the CSC niche) might contribute to the ovarian CSC phenotype (6). It is well known that metabolic pathways of cancer cells differ fundamentally from those of normal cells including altered energy-producing catabolic pathways such as glycolysis, glutaminolysis and oxidative phosphorylation, and energy-consuming anabolic pathways such as amino acid synthesis, pentose phosphate pathway, nucleotide synthesis and fatty acid synthesis (9). Metabolic rewiring in cancer cells results from both autonomous cell signaling via activated oncogenes and/or deactivated tumor suppressors and from adaptive changes in response to specific alterations in the tumor microenvironment. Persistent CSCs have to survive adverse host conditions such as lack of nutrients and oxygen, low pH and toxic milieu. Therefore, CSCs have to be armed with robust self-defense mechanisms and be able to recycle and generate many biologically relevant molecules in an autonomous manner. Accordingly, recent studies have linked anabolic processes such as de novo synthesis of fatty acids to the CSC phenotype, which consequently is characterized by overexpression of the corresponding rate-limiting enzyme fatty acid synthase (FASN). Intriguingly, inhibition of FASN has been shown to suppress growth of breast and glioma CSCs (10,11) and to cause downregulation of monounsaturated fatty acids (MUFA) in ovarian cancer (12,13).
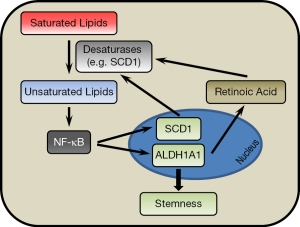
In the March 2017 issue of the journal Cell Stem Cell, Li et al. (14) published a paper applying hyperspectral-stimulated Raman scattering (SRS) microscopy for chemical imaging of living single cells. By combining this method with fluorescence-activated cell sorting (FACS), they were able to identify lipid unsaturation as a crucial feature of ovarian CSCs. The data were corroborated in more detail by mass spectrometry analysis. Flow-sorted CSCs expressing aldehyde dehydrogenase and CD133/prominin-1 (ALDH+/CD133+), were found to contain significantly higher levels of unsaturated lipids compared to non-CSCs (ALDH−/CD133−). Moreover, desaturation of lipids was significantly higher in ovarian cancer cells grown as spheroids compared to monolayer cultures. Increased lipid unsaturation in CSCs was directly associated with elevated expression of lipid desaturases. These enzymes were found to directly contribute to the maintenance of ovarian cancer cell stemness. Consequently, inhibition of lipid desaturases decreased the expression of ovarian CSC markers, impaired sphere formation in vitro and prevented tumorigenesis in vivo. Finally, the authors provide evidence that unsaturated fatty acids (UFA) can activate NF-κB thereby stimulating the expression of crucial stemness factors. In detail, Li et al. observed an increased total amount of lipid droplets containing high levels of unsaturated fatty acids in ALDH+/CD133+ ovarian CSC cells. Although lipid droplets are storage sites containing neutral lipids such as triacylglycerol, mass spectrometry revealed that the level of lipid unsaturation indicates specific changes in the overall lipid metabolic network of CSCs. Accordingly, marked increases in saturated and monounsaturated C16 and C18 fatty acids as well as in polyunsaturated C18, C20 and C22 fatty acids were suggesting intensified de novo lipogenesis in CSCs. This is consistent with previous findings demonstrating high levels of FASN and elevated FASN inhibitor sensitivity in CSCs (10,11). Controlled withdrawal or supplementation of exogenous fatty acids further supported the notion that CSCs rely on autonomous fatty acid synthesis rather than on exogenous lipid uptake. The FASN pathway generates saturated fatty acyl-CoA conjugates. These have to be further processed by downstream enzymes (desaturases), which introduce double bonds into the newly synthesized saturated carbon chains. Accordingly, ovarian CSCs contain high levels of fatty acid desaturases and reveal elevated sensitivity to desaturase inhibitors and small hairpin RNAs. Inhibition of desaturases caused downregulation of CSC markers such as ALDH1A1, Sox2, Nanog, and Oct-4 and abolished in vitro sphere formation and in vivo tumor initiation. When searching for a mechanistic link between lipid desaturation and the ovarian CSC phenotype, the NF-κB survival pathway, which has already been linked to cancer stemness (15,16), was identified as a crucial mediator. For example, inhibition of desaturases suppressed NF-κB transcriptional activity causing downregulation of known NF-κB target genes such as IL-6 and IL-8. Moreover, forced expression of the active subunit of NF-κB, p65 (relA), stimulated sphere formation and expression of the CSC marker ALDH1A1. Conversely, ALDH1A1 was downregulated upon pharmacological inhibition of NF-κB. Altogether, ovarian CSCs have high levels of FASN (10,11) and fatty acid desaturases (14), which make CSCs independent from external supply of lipids by stimulating de novo synthesis of saturated and unsaturated fatty acids. Lipid desaturation has been shown to activate NF-κB (17,18), albeit the mechanism for this interaction remains undefined. NF-κB, in turn, activates known target genes including the CSC marker ALDH1A1. This provides the link between NF-κB and stemness. Most intriguingly, Li et al. also demonstrate that NF-κB upregulates ∆9 desaturase [stearoyl-CoA desaturase 1 (SCD1)]—the enzyme that converts saturated into monounsaturated fatty acyl chains by introduction of one double bond. They conclude that NF-κB supports a positive feedback circuit by stimulating the stemness marker ALDH1A1 and the desaturating enzyme SCD1, which provides unsaturated lipids that in turn activate NF-κB (Figure 1). Moreover, data suggest an additional role of the CSC marker ALDH1A1 in the regulation of lipid desaturation mediated through differentiation factors such as retinoic acid.
While signaling pathways maintaining stemness of cancer cells have been elucidated in many experimental settings, knowledge about the role of the metabolic network in regulation of the CSC phenotype is still rudimentary. Therefore, work presented herein essentially contributes to our understanding of the metabolic phenotype of CSCs. Recent evidence demonstrates that mammary cancer cells undergoing epithelial-mesenchymal transition (EMT) develop a hypoxia-resistant CSC phenotype that prefers glycolysis and reduces the production of reactive oxygen species (19). This concurs with present data on CSCs’ autonomous production of MUFA-rich lipids and suggests that the CSCs develop a metabolic self-sufficient state rendering the cells independent from microenvironmental conditions. In addition, CSCs activate detoxifying enzymes such as ALDH conferring chemoresistance. Here the authors apply standard FACS procedures to enrich for ALDH+/CD133+ ovarian CSCs followed by microscopic evaluation under monochromatic light for quantitative determination of the hyperspectral-stimulated Raman scattering SRS shifts, which can discriminate between single and double bonds in the carbon backbone of fatty acyl chains in the lipid droplets of the cells. While this approach is still in an experimental stage the authors plan to combine both technologies (FACS and SRS) in one semi-automated procedure, which should be suitable for large scale routine applications. Thereby, a diagnostic approach using several functional markers for direct identification and separation of CSCs should become feasible. The data presented in the paper of Li et al. (14) demonstrate that lipid desaturases may be promising targets for drug-mediated elimination of CSCs. It is, however, surprising to see that ovarian CSCs strictly depend on intracellular lipid biosynthesis and are unable to use exogenous lipids, although it has previously been shown that ovarian cancer cells take up lipids through an FABP-4-mediated shuttling mechanism (20). On the other hand, knockout of SCD-1 induced various mechanism-based developmental aberrations in a mouse model (21). This raises concerns over the applicability of desaturase inhibitors for clinical use.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Duo Liu (Harbin Medical University Cancer Hospital, Harbin Medical University, Harbin, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.03.76). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Banks E, Beral V, Reeves G. The epidemiology of epithelial ovarian cancer: a review. Int J Gynecol Cancer 1997;7:425-38. [Crossref]
- Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLoS Med 2009;6:e1000114 [Crossref] [PubMed]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- SEER Stat Fact Sheets: Ovary Cancer. Available online: http://seer.cancer.gov/statfacts/html/ovary.html. Retrieved on March 03, 2017
- Rahman M, Deleyrolle L, Vedam-Mai V, et al. The cancer stem cell hypothesis: failures and pitfalls. Neurosurgery 2011;68:531-45. [Crossref] [PubMed]
- Garson K, Vanderhyden BC. Epithelial ovarian cancer stem cells: underlying complexity of a simple paradigm. Reproduction 2015;149:R59-70. [Crossref] [PubMed]
- Valent P, Bonnet D, De Maria R, et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer 2012;12:767-75. [Crossref] [PubMed]
- Nguyen LV, Vanner R, Dirks P, et al. Cancer stem cells: an evolving concept. Nat Rev Cancer 2012;12:133-43. [PubMed]
- Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 2012;21:297-308. [Crossref] [PubMed]
- Pandey PR, Okuda H, Watabe M, et al. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat 2011;130:387-98. [Crossref] [PubMed]
- Yasumoto Y, Miyazaki H, Vaidyan LK, et al. Inhibition of fatty acid synthase decreases expression of stemness markers in glioma stem cells. PLoS One 2016;11:e0147717 [Crossref] [PubMed]
- Veigel D, Wagner R, Stübiger G, et al. Fatty acid synthase is a metabolic marker of cell proliferation rather than malignancy in ovarian cancer and its precursor cells. Int J Cancer 2015;136:2078-90. [Crossref] [PubMed]
- Wagner R, Stübiger G, Veigel D, et al. Multi-level suppression of receptor-PI3K-mTORC1 by fatty acid synthase inhibitors is crucial for their efficacy against ovarian cancer cells. Oncotarget 2017;8:11600-13. [PubMed]
- Li J, Condello S, Thomes-Pepin J, et al. Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell 2017;20:303-14. e5.
- Shostak K, Chariot A. NF-kB, stem cells and breast cancer: the links get stronger. Breast Cancer Res 2011;13:214. [Crossref] [PubMed]
- Jia D, Yang W, Li L, et al. β-Catenin and NF-κB co-activation triggered by TLR3 stimulation facilitates stem cell-like phenotypes in breast cancer. Cell Death Differ 2015;22:298-310. [Crossref] [PubMed]
- Camandola S, Leonarduzzi G, Musso T, et al. Nuclear factor κB is activated by arachidonic acid but not by eicosapentaenoic acid. Biochem Biophys Res Commun 1996;229:643-7. [Crossref] [PubMed]
- Poletto AC, Furuya DT, David-Silva A, et al. Oleic and linoleic fatty acids downregulate Slc2a4/GLUT4 expression via NFKB and SREBP1 in skeletal muscle cells. Mol Cell Endocrinol 2015;401:65-72. [Crossref] [PubMed]
- Dong C, Yuan T, Wu Y, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell 2013;23:316-31. [Crossref] [PubMed]
- Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 2011;17:1498-503. [Crossref] [PubMed]
- Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr 2001;131:2260-8. [PubMed]


