
What can we learn from 3 phase III trials of ASCEND-4: ceritinib vs. platinum/pemetrexed with pemetrexed maintenance, PROFILE 1004: crizotinib vs. platinum/pemetrexed, and J-ALEX: alectinib vs. crizotinib?
The echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) fusion gene is observed in 4–5% of non-small cell lung cancer (NSCLC) (1). The first-generation ALK-targeted tyrosine kinase inhibitor (TKI), crizotinib, showed significantly better objective responses rates (ORR) and longer progression free survival (PFS) than the standard platinum doublet chemotherapy in those patients (median PFS: 10.9 vs. 7.0 months, respectively; hazard ratio for progression or death with crizotinib: 0.45; ORR: 74% vs. 45%, respectively) (PROFILE 1014) (2). Now, ALK-TKI is considered as the first line standard treatment for patients with newly diagnosed advanced ALK-fusion gene positive NSCLC. Ceritinib is a next-generation, selective ALK inhibitor, approximately 28 to 39 times more potent than crizotinib against ALK-positive NSCLC cells. Moreover, ceritinib overcame several acquired crizotinib-resistant ALK mutations in vitro and in vivo (3).
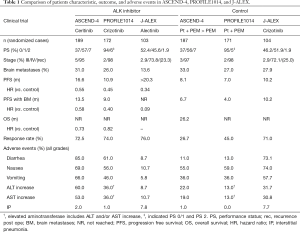
In the recent article by Soria et al. (4), the efficacy, safety, and patient-reported outcomes of ceritinib versus platinum plus pemetrexed doublet chemotherapy with pemetrexed maintenance in chemotherapy naïve patients with advanced ALK-positive NSCLC were evaluated. This is a randomized, open-label, global, phase III study in which 134 institutions from 28 countries participated. All eligible patients were histologically or cytologically confirmed advanced or metastatic non-squamous NSCLC with ALK-rearrangement determined centrally by VENTANA anti-ALK (D5F3) immunohistochemistry assay. Treatment schedule was determined either daily ceritinib (750 mg) in the ceritinib arm or platinum (cisplatin: 75 mg/m2 or carboplatin: AUC of 5–6) plus pemetrexed (500 mg/m2) given every 21 days for four cycles followed by pemetrexed maintenance every 21 days if not progressive disease in the chemotherapy group. Primary endpoint was PFS. Median PFS was 16.6 months [95% confidence interval (CI): 12.6–27.2] in the ceritinib arm and 8.1 months (95% CI: 5.8–11.1) in the platinum/pemetrexed followed by pemetrexed arm [hazard ratio 0.55; 95% CI: 0.42–0.73; P<0.00001] (Table 1). Thus, ceritinib has been positioned as the first-line treatment in patients with advanced ALK-rearranged NSCLC. ASCEND-4 is the first study that a next-generation ALK-TKI demonstrated the superiority in PFS compared with platinum/pemetrexed doublet chemotherapy with pemetrexed maintenance.
Full table
After the PROFILE 1014 study started from January 2011, the regimen of cisplatin/pemetrexed followed by pemetrexed has been established as the standard of care for the non-squamous NSCLC patients with good performance status (PS) (5,6). With regard to this point, ASCEND-4 study represents a valid comparator against the use of chemotherapy in the real clinical setting in this era. As considered in the results of the PROFILE 1014 and the ASCEND-4 studies, we strongly confirmed ALK-TKI as the standard treatment for the first-line treatment against ALK-positive advanced NSCLC patients. In addition, ceritinib is only ALK-TKI, which was compared with current standard chemotherapy including pemetrexed maintenance.
Because of the high blood-brain barrier permeability of ceritinib (7), patients with asymptomatic or stable brain metastases were included and the assessment of the intracranial treatment responses was pre-planned in ASCEND-4. In the prior phase I study of ceritinib, the remarkable intracranial responses and duration of response in patients with chemotherapy naïve and post-treatment with ALK-TKI were observed (ORR: 63% and 36%; duration of response: 8.2 and 11.1 months, respectively) (8). Intracranial efficacy of first-line crizotinib (n=39) versus chemotherapy (n=40) in patients with treated brain metastases in PROFILE 1014 was extensively analyzed (9). ORR (77% vs. 28%; P<0.001), median PFS (9.0 vs. 4.0 months; P<0.001), and median intracranial intent-to treat (15.7 vs. 12.5 months; P=0.063) seem to be advantageous with crizotinib arm. Although crizotinib is efficacious in patients with brain metastases, newer ALK-TKIs including ceritinib, alectinib (10), brigatinib (11), and lorlatinib (12) seem to show better clinical response in the intracranial metastases (13). In ASCEND-4 study, overall intracranial response rate in patients with measurable baseline metastases 72.7% in the ceritinib arm and 27.3% in the platinum/pemetrexed with pemetrexed maintenance arm (hazard ratio: 0.58) (4). Although it remains unclear whether maintenance with pemetrexed was effective for suppression of brain metastasis compared to no maintenance, continuous treatment of pemetrexed inhibited brain metastasis after stereotactic irradiation (14). A randomized trial (alectinib vs. crizotinib) in Japanese ALK-TKI-naïve patients with ALK-positive NSCLC were recently reported to show longer PFS (>20.3 months) in alectinib arm than (10.2 months) in crizotinib arm (Table 1) (15). Hazard ratio of PFS in patients with brain metastases is 0.09 although there was an imbalance between patient numbers with them (13.6% in alectinib arm and 27.9% in crizotinib arm) (Table 1). We learned that intracranial activity of ceritinib and crizotinib are better than that of chemotherapy. In addition, alectinib (and maybe ceritinib) shows better outcome regarding response and PFS than crizotinib in ALK-positive patients with brain metastases.
Most adverse events observed in ASDEND-4 study seemed mild and tolerable. Gastrointestinal toxicity including diarrhea, nausea, and vomiting in the ceritinib arm was higher than chemotherapy arm but most events were grade 1–2 (Table 1). Only 5% in ceritinib arm discontinued treatment due to adverse events. We may treat patients experiencing such toxicities with dose reduction (450 or 600 mg/day) or treatment interruption (16). Aminotransferases seemed to increase in ceritinib arm (ASCEND-4) and in crizotinib group (PROFILE 1014 and J-ALEX) (Table 1). Although incidence of interstitial pneumonia (around 8%) was high in both crizotinib and alectinib arms in J-ALEX study, drug-induced interstitial pneumonia often occurred in Japanese compared with other ethnicity (17). Thus, we cannot tell whether incidence of interstitial pneumonia by ceritinib was lower than that of crizotinib and alectinib. What we learned is that alectinib seems to have fewer adverse events compared with crizotinib and ceritinib.
The next-generation ALK-TKIs for the ALK-positive patients have been still performed in phase III settings: (I) NCT02075840 (ALEX), alectinib vs. crizotinib for systemic treatment-naïve patients in US; (II) NCT02373501 (ALTA-1L), brigatinib vs. crizotinib for patients who received up to 1 prior chemotherapy regimen and no previous TKIs including ALK-TKIs; (III) NCT02767804 (eXalt3), ensartinib vs. crizotinib for patients who received up to 1 prior chemotherapy regimen and no prior ALK-TKIs. Lorlatinib is now investigated in ALK-positive NSCLC patients who are treatment naïve or were treated with ALK-TKIs (NCT01970865, NCT02927340). Subsequently, the phase III study comparing lorlatinib with crizotinib in advanced ALK-positive patients without prior systemic NSCLC treatment just started on April 14, 2017 (NCT03052608). These results will help us to develop more efficient and less harmful treatment against ALK-positive NSCLC.
Long-term survival and ultimately cure without quality of life impairment in advanced ALK-positive NSCLC patients are our goals. Approaches to overcome the resistance using newer ALK-TKIs, cytotoxic agents, angiogenesis inhibitors, and immunocheckpoint inhibitors have also been performed in clinical trials (18). In the point of view of long-term overall survival, the benefit of experimental arms in ASCEND-4, PROFILE 1014, and J-ALEX studies has not been clarified. Thus, cytotoxic chemotherapy including pemetrexed and first-generation ALK-TKI such as crizotinib may be useful in first-line treatment because resistance mechanisms and therapeutic strategies overcoming resistance have been elucidated (19,20). Researchers are sincerely engaged in such translational and clinical studies, which will offer hope patients with the intractable disease (21-23).
Acknowledgments
Funding: This work was partly supported by JSPS KAKENHI Grant Number JP26830118 (NO).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Shaohua Cui (Department of Pulmonary Medicine, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China).
Conflicts of Interest: N Takigawa has received research funds and honoraria from Pfizer Inc., Japan, Chugai Pharmaceutical, Novartis Pharmaceutical, and Ili lilly Japan. N Ochi has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res 2008;14:6618-24. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 2014;4:662-73. [Crossref] [PubMed]
- Soria JC, Tan DS, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247-55. [Crossref] [PubMed]
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:2895-902. [Crossref] [PubMed]
- Zykadia prescribing information. Basel: Novartis AG, 2014. Available online:https://www.pharma.us.novartis.com/product/pi/pdf/zykadia.pdf (accessed March 19, 2017).
- Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452-63. [Crossref] [PubMed]
- Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial Efficacy of Crizotinib Versus Chemotherapy in Patients With Advanced ALK-Positive Non-Small-Cell Lung Cancer: Results From PROFILE 1014. J Clin Oncol 2016;34:2858-65. [Crossref] [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [Crossref] [PubMed]
- Camidge DR, Bazhenova L, Salgia R, et al. First-in-human dose-finding study of the ALK/EGFR inhibitor AP26113 in patients with advanced malignancies: Updated results. J Clin Oncol 2013;31:abstr 8031.
- Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem 2014;57:4720-44. [Crossref] [PubMed]
- Burns MW, Kim ES. Profile of ceritinib in the treatment of ALK+ metastatic non-small-cell lung cancer. Lung Cancer (Auckl) 2015;6:35-42. [PubMed]
- Ochi N, Yamane H, Yamagishi T, et al. Continuous pemetrexed treatment for brain metastasis in non-small cell lung cancer--a report of two cases. Lung Cancer 2013;80:111-3. [Crossref] [PubMed]
- Nokihara T, Hida T, Kondo M, et al. Alectinib (ALC) versus crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK+ NSCLC): Primary results from the J-ALEX study. J Clin Oncol 2016;34:abstr 9008.
- Dziadziuszko R, Kim DW, Bearz A, et al. Phase 1 study of ceritinib 450 mg or 600 mg taken with a low-fat meal versus 750 mg in fasted state in ALK+ metastatic NSCLC. Presented at the World Conference on Lung Cancer; Vienna, Austria; Dec 4-7, 2016. Abstract number P3.02a-036.
- Takeuchi T, Kameda H. The Japanese experience with biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol 2010;6:644-52. [Crossref] [PubMed]
- Isozaki H, Takigawa N, Kiura K. Mechanisms of Acquired Resistance to ALK Inhibitors and the Rationale for Treating ALK-positive Lung Cancer. Cancers (Basel) 2015;7:763-83. [Crossref] [PubMed]
- Zhang D, Ochi N, Takigawa N, et al. Establishment of pemetrexed-resistant non-small cell lung cancer cell lines. Cancer Lett 2011;309:228-35. [Crossref] [PubMed]
- Lin JJ, Riely GJ, Shaw AT. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov 2017;7:137-55. [Crossref] [PubMed]
- Isozaki H, Ichihara E, Takigawa N, et al. Non-Small Cell Lung Cancer Cells Acquire Resistance to the ALK Inhibitor Alectinib by Activating Alternative Receptor Tyrosine Kinases. Cancer Res 2016;76:1506-16. [Crossref] [PubMed]
- Isozaki H, Hotta K, Ichihara E, et al. Protocol Design for the Bench to Bed Trial in Alectinib-Refractory Non-Small-Cell Lung Cancer Patients Harboring the EML4-ALK Fusion Gene (ALRIGHT/OLCSG1405). Clin Lung Cancer 2016;17:602-5. [Crossref] [PubMed]
- Katayama R. Therapeutic strategies and mechanisms of drug resistance in anaplastic lymphoma kinase (ALK)-rearranged lung cancer. Pharmacol Ther 2017; [Epub ahead of print]. [Crossref] [PubMed]


