
RIPK1 and allies in the battle against hepatocyte apoptosis and liver cancer
Introduction
Liver cancer constitutes the third most frequent cause for cancer-related deaths worldwide. It typically appears in individuals with underlying liver diseases, which most commonly develop in the context of chronic infections with hepatitis viruses, alcoholic and non-alcoholic steatohepatitis or aflatoxin-mediated toxicity (1,2). Continuous death of hepatocytes is a central event in these liver pathologies and leads to a regenerative response characterized by hepatocyte proliferation, inflammation, steatosis and fibrosis that can progress to cirrhosis. This setting becomes a predisposing factor for the development of hepatocellular cancer (HCC), the most frequent form of primary liver cancer (3,4). Understanding the molecular mechanisms governing hepatocellular death is therefore crucial for designing preventive therapies.
In recent years, receptor interacting protein kinase 1 (RIPK1) has emerged as a key determinant of cell decisions to live or die by regulating pathways leading to cell survival or programmed cell death (5-7). Although RIPK1 plays a critical role downstream of many death receptors (DRs) and inflammatory signaling pathways, its functions have been best characterized in tumor necrosis factor (TNF) signaling cascade (Figure 1). Upon activation by TNF, TNF receptor 1 (TNFR1) undergoes trimerization and mediates the formation of a cytoplasmic complex (Complex I) by recruiting TNFR1 associated death domain protein (TRADD) and RIPK1. This leads to recruitment of TNF receptor-associated factor (TRAF2), the cellular inhibitors of apoptosis 1 and 2 (cIAP1/2) and the linear ubiquitin chain assembly complex (LUBAC). Several ubiquitination events are associated with RIPK1 and other Complex I components influencing the downstream functional outputs. First, cIAP1/2 mediate K11-, K48- and K63-linked ubiquitination of RIPK1 and cIAP1 itself allowing the recruitment of TAK1 (through its interacting proteins TAB2/3) and LUBAC subunits (HOIP, HOIL-1 and Sharpin), respectively. In turn, LUBAC adds M1-linked (linear) ubiquitin chains on RIPK1 and the NF-κB essential modulator (NEMO) (8). NEMO is the regulatory subunit of the IKK complex, which also includes IKK1/IKKα and IKK2/IKKβ kinases. The IKK complex is best known for mediating the activation of NF-κB signaling by phosphorylating the Inhibitor of NF-κB (IκB) family members, thus promoting their K48 ubiquitination and proteasomal degradation. This allows dimers of NF-κB transcription factors (RelA/p65, RelB, c-Rel, p50/NF-κB1 and p52/NF-κB2) to enter the nucleus and regulate the expression of proinflammatory but also pro-survival genes (9). More recently, NF-κB-independent, pro-survival functions of the IKK complex have also been described (10).
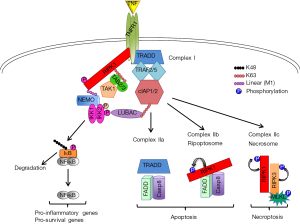
Destabilization of Complex I due to NF-κB inhibition or disruptions in its ubiquitin landscape results in the formation of distinct intracellular complexes that can induce cell death (Figure 1). Complex IIa, which predominantly forms upon NF-κB inhibition, involves the TRADD-dependent interaction between FADD and Caspase-8 and induces apoptosis. Complex IIb (or ripoptosome) comprises RIPK1, FADD and Caspase-8 and forms when RIPK1 ubiquitination (and possibly phosphorylation) is disrupted. Its formation is RIPK1 kinase activity-dependent and it also mediates apoptosis. When Caspase-8 is inhibited (upon FADD or Caspase-8 deficiency or treatment with the pan-caspase inhibitor zVAD-fmk), cells can form an alternative Complex IIb (or Complex IIc or necrosome) that is RIPK1 kinase activity-dependent and includes RIPK3 and its downstream target mixed lineage kinase-like (MLKL). Activation of this complex induces necroptosis and its formation appears to be cell type-specific depending on the expression levels of RIPK3 and MLKL (5-7,10,11).
RIPK1 is dispensable for normal liver development
RIPK1 is recognized as a protein with “two faces”: one promoting cell survival through its scaffolding properties and one favoring cell death through its kinase activity (12). This was originally supported from the fact that RIPK1 constitutive knockout mice die perinatally (13-16), while mice expressing a kinase inactive form of RIPK1 (RIPK1D138N or RIPK1K45A) are viable and show resistance to TNF-induced inflammatory pathologies and necroptosis induction in vitro (17-19). Along this line, intestinal- and epidermis-specific deletion of RIPK1 leads to spontaneous death of epithelial cells and development of intestinal and skin inflammation (20,21). In sharp contrast, mice with liver parenchymal cell (LPC)-specific deletion of RIPK1 (RIPK1LPC-KO) do not exhibit substantial spontaneous liver damage, suggesting that RIPK1 is dispensable for liver homeostasis during development (22-27). This difference implies that hepatocytes may use redundant/multiple survival mechanisms that compensate for the lack of RIPK1. Alternatively, the stimuli that can trigger hepatocyte death in the absence of RIPK1 could be absent or below a critical threshold during organ development. Several genetic mouse models, such as NEMOLPC-KO, IKK1/2LPC-KO or TAK1LPC-KO mice (22,28-30), were shown to develop spontaneous liver damage suggesting that death-inducing stimuli are present during liver development, and therefore, the presence of compensatory survival mechanisms operating in hepatocytes is likely the critical factor.
The “two-faced” behavior of RIPK1 in liver disease pathogenesis has been genetically demonstrated in NEMOLPC-KO mice, which show spontaneous hepatocyte apoptosis resulting in strong regenerative response, including hepatitis, and eventually in HCC formation (22,28). Expression of a kinase-inactive RIPK1D138N mutant in NEMO-deficient hepatocytes significantly prevented spontaneous apoptosis, chronic liver pathology and HCC (22). The pro-apoptotic role of RIPK1 kinase activity has also been shown in cells depleted for cIAP1/2 using SMAC mimetics (SM) (31), or in TAK1- (32), IKK1/IKK2- (33) and LUBAC-deficient mouse embryonic fibroblasts (MEFs) (19). Conversely, complete absence of RIPK1 did not prevent apoptosis of NEMO-deficient or IKK1/2-deficient hepatocytes (22,23) underscoring the importance of RIPK1’s scaffolding functions. In the absence of RIPK1, TRADD mediates an alternative pro-apoptotic pathway in hepatocytes, as NEMO/RIPK1/TRADDLPC-KO mice showed no spontaneous liver damage (22). Both TRADD and RIPK1 interact with Caspase-8 upon TNF stimulation, but the presence of two independent complexes has not been demonstrated (34). While the presence of TRADD in Complex IIa has been inferred by studies showing that TRADD counteracts RIPK1-induced cell death (31,35), an interaction of TRADD with FADD or Caspase-8 was not observed in MEFs treated with TNF/Cycloheximide that induces TRADD-mediated apoptosis (33). Therefore, the precise molecular role of TRADD in the alternative apoptosis-inducing pathway remains elusive.
RIPK1 is indispensable for survival in mouse models of acute liver injury
In contrast to the lack of spontaneous liver phenotype in RIPK1LPC-KO mice, mounting evidence show that the presence of RIPK1 in hepatocytes is essential for preventing acute hepatocellular damage in several in vivo models. Upon LPS- or CpG-DNA-induced liver damage, resident macrophages (Kupffer cells) become strongly activated and secrete high levels of TNF that initiates TNFR1 signaling cascade in hepatocytes (25,36). RIPK1LPC-KO mice were shown to be highly sensitive to these stimuli and undergo apoptosis (25,27,37). Similarly, RIPK1LPC-KO mice were greatly susceptible to apoptosis induced by Concanavalin-A (ConA) (24), a model of TNFR1-dependent, T cell-mediated autoimmune hepatitis (36). RIPK1 knock-down using anti-sense oligos had also a detrimental effect in mice injected with α-galactosyl ceramide (αGalCer), a liver injury model depending on the release of various cytokines, including TNF, by activated NKT cells (26). Finally, the impact of RIPK1 in acetaminophen (APAP)-induced hepatotoxicity, a TNF-independent acute liver injury model, has been more controversial. While RIPK1LPC-KO mice were as sensitive to APAP toxicity as their wild-type controls (38), treatment of mice with RIPK1 anti-sense oligonucleotides significantly prevented liver damage and increased overall survival (39). Side-effects of using oligos, RIPK1 functions in non-parenchymal cells or adaptive responses of hepatocytes lacking RIPK1 already in utero have been proposed as arguments for explaining the opposite results (38,39). Additionally, RIPK1 depletion by the anti-sense oligos may not have been strong enough to trigger the TRADD-mediated apoptotic pathway that has been shown to operate in RIPK1-deficient hepatocytes (22,37).
Contrary to RIPK1 scaffolding function, the enzymatic activity of RIPK1 was also shown to play a pro-apoptotic role in acute liver injury models. Inhibition of RIPK1 kinase activity by Nec-1 or Nec-1s, a more specific inhibitor, and genetic expression of RIPK1D138N or RIPK1K45A significantly protected mice from APAP- (39,40), αGalCer- (26) and ConA-induced liver damage (41), though one study also reported exacerbated liver injury upon Nec-1 treatment in the later model (40). Another recent study also showed that RIPK1K45A mice were completely protected from hepatocyte apoptosis induced by TNF and the IKK1/2 inhibitor, TPCA-1, whereas no protection was observed upon injection of TNF and D-galactosamine (inhibitor of hepatocyte gene transcription) (33), suggesting that RIPK1 kinase activity mainly mediates the NF-κB-independent pro-apoptotic pathway (see below).
Interplay between RIPK1, IKK complex and NF-κB
The resistance of RIPK1-deficient hepatocytes to spontaneous cell death during normal liver development implies RIPK1’s close crosstalk with other key molecules/pathways regulating cell survival. The interplay of RIPK1 and NF-κB pathway is of particular interest. The importance of both NF-κB-dependent and independent mechanisms for spontaneous liver disease has been suggested in NEMOLPC-KO mice. While NF-κB deficiency in RelA/RelB/cRelLPC-KO (NF-κBLPC-KO) mice did not recapitulate the pathology of NEMOLPC-KO mice, the expression of a constitutively active form of IKK2 rescued the spontaneous apoptosis observed in NEMOLPC-KO mice largely by activating canonical NF-κB signaling (22). This dual (NF-κB-dependent and independent) pro-survival function of NEMO is not only hepatocyte-specific, as similar results have been reported in intestinal epithelial cells (42) and Jurkat T cells (43). These findings are also consistent with the previously described existence of two distinct apoptotic pathways in cancer cells, an NF-κB/cFLIP-dependent and an NF-κB-independent but RIPK1/cIAP-dependent pathway (31). In a “two-hit model”, simultaneous inhibition of RIPK1 scaffolding functions and NF-κB signaling would be required to induce spontaneous liver damage and chronic liver disease (Figure 2).
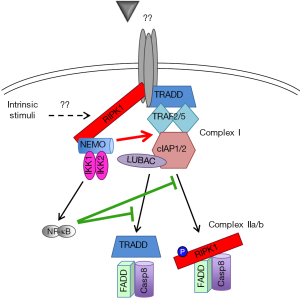
Schneider et al. recently provided additional support to this notion by showing that RIPK1/TRAF2LPC-KO mice develop spontaneous liver damage, cholestasis, chronic hepatitis, fibrosis and HCC. Unlike the respective single knockout cells, RIPK1/TRAF2-deficient hepatocytes exhibited impaired canonical NF-κB signaling (27). RIPK1 was originally shown to be required for TNF-induced NF-κB activation (13), but this finding was subsequently challenged (44) and several studies have shown robust LPS/TNF-induced NF-κB activation in RIPK1-deficient hepatocytes (24,26,27). On the other hand, TRAF2-deficient hepatocytes can sustain NF-κB activation possibly due to the redundant function of TRAF5 (45). The presence of two independent pro-survival pathways is also supported by the fact that both NF-κBLPC-KO and RIPK1LPC-KO mice were susceptible to LPS-induced liver damage (22,27). Curiously, Schneider et al. show that although LPS/TNF stimulation induces rapid TRAF2 downregulation in RIPK1-deficient hepatocytes, this did not impair their ability to activate NF-κB, unlike what was observed in RIPK1/TRAF2-deficient hepatocytes (27). This implies that when the death-inducing stimulus is above a certain threshold, it can cause substantial liver damage even when NF-κB signaling is not significantly inhibited. Based on the proposed “two-hit model” (Figure 2), one would predict that combined ablation of RIPK1 and NF-κB signaling would culminate in stronger LPS-induced acute liver damage compared to the single knockouts.
Schneider et al. also generated TRAF2/IKK2LPC-KO mice as a way to genetically simulate their molecular findings in RIPK1/TRAF2LPC-KO mice. These mice showed spontaneous liver damage, albeit less strong than in RIPK1/TRAF2LPC-KO mice and without exhibiting cholestasis. One-year-old TRAF2/IKK2LPC-KO mice developed HCC but of much smaller size than RIPK1/TRAF2 LPC-KO mice (27), likely reflecting the lower hepatocellular damage and lack of cholestasis in the former mice. Although the LPC-specific IKK2 deficiency was used to exemplify the contribution NF-κB blockade in the liver phenotype of RIPK1/TRAF2 LPC-KO mice, it is worth mentioning that Luedde et al have shown that IKK2 deficiency does not fully block NF-κB activation in hepatocytes due to a partial compensation by IKK1 (29). More importantly, RIPK1 has been identified as a phosphorylation substrate of IKK1/2 in MEFs and hepatocytes (23,33). This RIPK1 phosphorylation was shown to have an NF-κB-independent, prosurvival function. In MEFs, RIPK1 phosphorylation was abolished in the absence of IKK1/2, NEMO, TAK1 or cIAP1/2 suggesting that the event takes place at complex I (33). On the other side, the liver study showed that RIPK1 phosphorylation was inhibited in IKK1/2-deficient but not in NEMO-deficient hepatocytes (23). However, how IKK1/2 would be able to associate with and catalyze RIPK1 phosphorylation in the absence of NEMO remains unclear. The biological significance of this pro-survival, IKK-mediated RIPK1 phosphorylation will need to be validated using suitable RIPK1 mutants in in vivo mouse models.
Formal proof that the canonical NF-κB signaling and RIPK1 act synergistically to prevent spontaneous apoptosis in hepatocytes has come from a recent study reporting that RIPK1/RelALPC-KO mice develop spontaneous apoptosis, chronic liver disease and eventually HCC (37), as NEMOLPC-KO and TRAF2/IKK2LPC-KO mice do (22,27).
RIPK1 prevents hepatocyte apoptosis by stabilizing TRAF2 and cIAP1
Another important issue is how RIPK1 affects other components of Complex I to prevent apoptosis. The stability of cIAP1/2 and TRAF2 appear to be critical events for the sensitization to apoptosis, as they were downregulated in NEMO-deficient hepatocytes (22). The same was true for cIAP1 in liver lysates from RIPK1/TRAF2LPC-KO mice (27). cIAP1 degradation correlated with Caspase-8 activation in Complex IIb both in NEMOLPC-KO and RIPK1/TRAF2LPC-KO mice (22,27), as previously described (46). Accordingly, both TRAF2 and cIAP1 were shown to undergo TNF-induced proteasomal degradation in RIPK1−/− but not in WT MEFs (47).
An additional molecule that synergizes with RIPK1 to prevent cell death is cFLIP that negatively regulates apoptosis and necroptosis (12). cFLIP protein levels were strongly reduced in NEMO- and RIPK1/TRAF2-deficient hepatocytes (22,27). Interestingly, its levels remained low in RIPK1/TRAF2/Caspase-8LPC-KO mice, where apoptosis was prevented, and this was attributed to the observed NF-κB inhibition (27). However, this is unlikely because cFLIP levels were restored in NEMOLPC-KO/RIPK1D138N mice, where apoptosis was strongly prevented but NF-κB was still inhibited due to the lack of NEMO (22). Unlike cancer cells where cFLIP is a typical NF-κB-responsive gene (48), its mRNA expression in NEMO- or NF-κB-deficient hepatocytes was not significantly affected. Thus, its protein levels at least in primary hepatocytes are likely regulated by additional NF-κB-independent transcription factors and post-translational mechanisms.
Different stimuli induce spontaneous and acute liver damage
Contrary to most of the acute liver injury models, where the cell death stimulus is defined, what triggers the spontaneous death of hepatocytes in all genetic mouse models of chronic liver disease leading to HCC is still unresolved. Considering that deletion of FADD or Caspase-8 prevents hepatocytes apoptosis in most of these mice (27,28,49,50), one would expect that death ligands that initiate extrinsic apoptotic pathways are important. Surprisingly, combined LPC-specific deletion of TNFR1, TRAILR and Fas did not protect NEMO-deficient hepatocytes from spontaneous death in vivo (49). Likewise, TNFR1 deletion in LPCs did not ameliorate the liver pathology in RIPK1/RelALPC-KO mice (37). The same was recently shown in HOIPLPC-KO mice that develop a spontaneous liver pathology as NEMOLPC-KO mice (51). This raises the question whether any of the remaining DRs (DR3 or DR6) may be involved (52). The most likely, however, is that low levels of multiple stimuli, including ligands activating DRs or Toll-like receptors, are the inducers of spontaneous liver damage. Moreover, intrinsic pathways inducing apoptosis could also contribute. For instance, unresolved ER stress was shown to induce DR-independent, RIPK1- and Caspase-8-dependent apoptosis in MEFs (53). Additionally, TAK1/NEMO/RIPK1-mediated activation of NF-κB signaling is shown to be part of the DNA damage response (54,55), while a DR-independent formation of Complex IIb/Ripoptosome has been described upon degradation of cIAPs induced by genotoxic stress (56,57). The multiple cell divisions that hepatocytes undergo during liver organogenesis in the first weeks after birth (when spontaneous apoptosis first occurs) could induce replicative stress leading to activation of analogous signaling pathways (58).
Translational aspects for human chronic liver disease and HCC patients
The type of programmed cell death has been associated with the elicited immunological responses thereby potentially affecting cancer development (5). While multiple mouse studies have demonstrated the relevance of apoptosis for liver disease development (22,25-28,37,49,50), the significance of hepatocyte necroptosis remains controversial (3,59). The absence or minimal expression of RIPK3, a bona-fide regulator of necroptosis, could explain why hepatocytes are resistant to necroptosis, while the positive or negative effect that RIPK3 KO mice showed in some liver injury models could reflect a role of RIPK3 in modulating necroptosis or inflammation in a non-LPC compartment [see (59) for a critical review].
Undoubtedly, the aforementioned mouse studies demonstrate a causal link between hepatocyte death and chronic steatohepatitis, which in turn promotes HCC development. These data highlight the potential benefit of targeting apoptosis (as the most relevant cell death type) in chronic liver disease patients as a preventive approach against HCC development. Accordingly, the pan-caspase inhibitor IDN-6556 is currently in clinical trials in such patients. Additionally, RIPK1 kinase inhibitors could also be efficient in preventing apoptosis in at least a subset of patients, and are likely to be well tolerated considering the lack of phenotype in mice expressing kinase-inactive RIPK1.
Despite the association between cell death and liver disease pathogenesis, no clear role for RIPK1 and other Complex I-related molecules in HCC initiation or progression has been so far demonstrated. Two opposing studies using the DEN-induced hepatocarcinogenesis model in mice have shown a detrimental or beneficial effect on liver tumor burden in IKK2LPC-KO (60) and RIPK1LPC-KO mice (37), respectively. However, these effects were attributed to a differential impact of the cell death observed early after DEN injection rather than a late effect of the deleted genes in tumor progression.
Complex I members were also not found significantly enriched in large exome sequencing projects on human HCC samples (61,62). However, there are studies showing a correlation between the expression of certain molecules and HCC patient prognosis. Loss of NEMO (63) or combined loss of RIPK1/TRAF2 immunoreactivity (27) in human HCC samples was associated with poor overall patient survival. Moreover, downregulation of RIPK1 and IKK1/2 was reported in a significant percentage of human HCC biopsies, albeit no correlation was observed to the underlying disease etiologies (23). Considering the pro-survival role of these molecules, the benefit of their downregulation for cancer cells is counterintuitive. Most probably, the reduction in protein levels has taken place at an early pre-cancerous stage leading to increased hepatocyte apoptosis and promoting the underlying hepatitis. However, during HCC development, tumor-initiating hepatocytes must have upregulated anti-apoptotic mechanisms to overcome this deficit and avoid cell death in a microenvironment with increased death-inducing stimuli. Indeed, hepatobiliary cancer cells are often resistant to TRAIL-induced death because they overexpress pro-survival proteins and targeting cIAP1 was shown to restore sensitivity to TRAIL (46).
Taken together, reduced immunoreactivity for RIPK1, TRAF2 and IKK complex subunits could have a prognostic value for chronic liver disease patients and HCC patient survival, although more extensive studies will need to be performed in larger patient cohorts. Based on our understanding of the RIPK1-mediated signaling pathways, it might be beneficial for HCC patients with low RIPK1 immunoreactivity to use NF-κB or cIAP inhibitors in their therapeutic scheme, as these HCCs are likely to have upregulated such anti-apoptotic pathways. In this case, the challenge will certainly be to develop ways for delivering these drugs preferentially in liver cancer cells.
Acknowledgments
I would like to thank all members of the Pasparakis lab and in particular M. Pasparakis, T.M. Van and R. Schwarzer for their useful suggestions on the content of this manuscript.
Funding: My research has been supported by a Marie Curie Career Development Fellowship (Proposal No: 275767) and a World Cancer Research grant (Award No. 15-0228).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Zhen-Yu Lin, (Cancer center, Union hospital, Huazhong University of Science and Technology, Wuhan, China)
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.04.16). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shariff MI, Cox IJ, Gomaa AI, et al. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol 2009;3:353-67. [Crossref] [PubMed]
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. [Crossref] [PubMed]
- Guicciardi ME, Malhi H, Mott JL, et al. Apoptosis and necrosis in the liver. Compr Physiol 2013;3:977-1010. [PubMed]
- Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology 2014;147:765-83 e4.
- Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature 2015;517:311-20. [Crossref] [PubMed]
- Christofferson DE, Li Y, Yuan J. Control of Life-or-Death Decisions by RIP1 Kinase. Annu Rev Physiol 2014;76:129-50. [Crossref] [PubMed]
- Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol 2015;16:689-97. [Crossref] [PubMed]
- Peltzer N, Darding M, Walczak H. Holding RIPK1 on the Ubiquitin Leash in TNFR1 Signaling. Trends Cell Biol 2016;26:445-61. [Crossref] [PubMed]
- Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev 2012;26:203-34. [Crossref] [PubMed]
- Ting AT, Bertrand MJ. More to Life than NF-kappaB in TNFR1 Signaling. Trends Immunol 2016;37:535-45. [Crossref] [PubMed]
- Varfolomeev E, Vucic D. Intracellular regulation of TNF activity in health and disease. Cytokine 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Weinlich R, Green DR. The two faces of receptor interacting protein kinase-1. Mol Cell 2014;56:469-80. [Crossref] [PubMed]
- Kelliher MA, Grimm S, Ishida Y, et al. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 1998;8:297-303. [Crossref] [PubMed]
- Dillon CP, Weinlich R, Rodriguez DA, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell 2014;157:1189-202. [Crossref] [PubMed]
- Rickard JA, O'Donnell JA, Evans JM, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 2014;157:1175-88. [Crossref] [PubMed]
- Kaiser WJ, Daley-Bauer LP, Thapa RJ, et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci U S A 2014;111:7753-8. [Crossref] [PubMed]
- Newton K, Dugger DL, Wickliffe KE, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 2014;343:1357-60. [Crossref] [PubMed]
- Polykratis A, Hermance N, Zelic M, et al. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J Immunol 2014;193:1539-43. [Crossref] [PubMed]
- Berger SB, Kasparcova V, Hoffman S, et al. Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J Immunol 2014;192:5476-80. [Crossref] [PubMed]
- Takahashi N, Vereecke L, Bertrand MJ, et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature 2014;513:95-9. [Crossref] [PubMed]
- Dannappel M, Vlantis K, Kumari S, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 2014;513:90-4. [Crossref] [PubMed]
- Kondylis V, Polykratis A, Ehlken H, et al. NEMO Prevents Steatohepatitis and Hepatocellular Carcinoma by Inhibiting RIPK1 Kinase Activity-Mediated Hepatocyte Apoptosis. Cancer Cell 2015;28:582-98. [Crossref] [PubMed]
- Koppe C, Verheugd P, Gautheron J, et al. I kappa B Kinase alpha/beta Control Biliary Homeostasis and Hepatocarcinogenesis in Mice by Phosphorylating the Cell-Death Mediator Receptor-Interacting Protein Kinase 1. Hepatology 2016;64:1217-31. [Crossref] [PubMed]
- Filliol A, Piquet-Pellorce C, Le Seyec J, et al. RIPK1 protects from TNF-alpha-mediated liver damage during hepatitis. Cell Death Dis 2016;7:e2462 [Crossref] [PubMed]
- Filliol A, Piquet-Pellorce C, Raguenes-Nicol C, et al. RIPK1 protects hepatocytes from Kupffer cells-mediated TNF-induced apoptosis in mouse models of PAMP-induced hepatitis. J Hepatol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Suda J, Dara L, Yang L, et al. Knockdown of RIPK1 Markedly Exacerbates Murine Immune-Mediated Liver Injury through Massive Apoptosis of Hepatocytes, Independent of Necroptosis and Inhibition of NF-kappaB. J Immunol 2016;197:3120-9. [Crossref] [PubMed]
- Schneider AT, Gautheron J, Feoktistova M, et al. RIPK1 Suppresses a TRAF2-Dependent Pathway to Liver Cancer. Cancer Cell 2017;31:94-109. [Crossref] [PubMed]
- Luedde T, Beraza N, Kotsikoris V, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 2007;11:119-32. [Crossref] [PubMed]
- Luedde T, Heinrichsdorff J, de Lorenzi R, et al. IKK1 and IKK2 cooperate to maintain bile duct integrity in the liver. Proc Natl Acad Sci U S A 2008;105:9733-8. [Crossref] [PubMed]
- Bettermann K, Vucur M, Haybaeck J, et al. TAK1 Suppresses a NEMO-Dependent but NF-kappa B-Independent Pathway to Liver Cancer. Cancer Cell 2010;17:481-96. [Crossref] [PubMed]
- Wang L, Du FH, Wang XD. TNF-alpha induces two distinct caspase-8 activation pathways. Cell 2008;133:693-703. [Crossref] [PubMed]
- Dondelinger Y, Aguileta MA, Goossens V, et al. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ 2013;20:1381-92. [Crossref] [PubMed]
- Dondelinger Y, Jouan-Lanhouet S, Divert T, et al. NF-kappa B-Independent Role of IKK alpha/IKK beta in Preventing RIPK1 Kinase-Dependent Apoptotic and Necroptotic Cell Death during TNF Signaling. Mol Cell 2015;60:63-76. [Crossref] [PubMed]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 2003;114:181-90. [Crossref] [PubMed]
- Zheng L, Bidere N, Staudt D, et al. Competitive control of independent programs of tumor necrosis factor receptor-induced cell death by TRADD and RIP1. Mol Cell Biol 2006;26:3505-13. [Crossref] [PubMed]
- Wroblewski R, Armaka M, Kondylis V, et al. Opposing Role of Tumor Necrosis Factor Receptor 1 Signaling in T Cell-Mediated Hepatitis and Bacterial Infection in Mice. Hepatology 2016;64:508-21. [Crossref] [PubMed]
- Van TM, Polykratis A, Straub BK, et al. Kinase-independent RIPK1 functions regulate hepatocyte survival and liver carcinogenesis. J Clin Invest 2017; [Epub ahead of print].
- Schneider AT, Gautheron J, Tacke F, et al. Receptor Interacting Protein Kinase 1 (RIPK1) in Hepatocytes Does Not Mediate Murine Acetaminophen Toxicity. Hepatology 2016;64:306-8. [Crossref] [PubMed]
- Dara L, Johnson H, Suda J, et al. Receptor Interacting Protein Kinase 1 Mediates Murine Acetaminophen Toxicity Independent of the Necrosome and Not Through Necroptosis. Hepatology 2015;62:1847-57. [Crossref] [PubMed]
- Deutsch M, Graffeo CS, Rokosh R, et al. Divergent effects of RIP1 or RIP3 blockade in murine models of acute liver injury. Cell Death Dis 2015;6:e1759 [Crossref] [PubMed]
- Gunther C, He GW, Kremer AE, et al. The pseudokinase MLKL mediates programmed hepatocellular necrosis independently of RIPK3 during hepatitis. J Clin Invest 2016;126:4346-60. [Crossref] [PubMed]
- Vlantis K, Wullaert A, Polykratis A, et al. NEMO Prevents RIP Kinase 1-Mediated Epithelial Cell Death and Chronic Intestinal Inflammation by NF-kappa B-Dependent and -Independent Functions. Immunity 2016;44:553-67. [Crossref] [PubMed]
- Legarda-Addison D, Hase H, O'Donnell MA, et al. NEMO/IKKgamma regulates an early NF-kappaB-independent cell-death checkpoint during TNF signaling. Cell Death Differ 2009;16:1279-88. [Crossref] [PubMed]
- Wong WW, Gentle IE, Nachbur U, et al. RIPK1 is not essential for TNFR1-induced activation of NF-kappaB. Cell Death Differ 2010;17:482-7. [Crossref] [PubMed]
- Tada K, Okazaki T, Sakon S, et al. Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-kappa B activation and protection from cell death. J Biol Chem 2001;276:36530-4. [Crossref] [PubMed]
- Guicciardi ME, Mott JL, Bronk SF, et al. Cellular inhibitor of apoptosis 1 (cIAP-1) degradation by caspase 8 during TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. Exp Cell Res 2011;317:107-16. [Crossref] [PubMed]
- Gentle IE, Wong WW, Evans JM, et al. In TNF-stimulated cells, RIPK1 promotes cell survival by stabilizing TRAF2 and cIAP1, which limits induction of non-canonical NF-kappaB and activation of caspase-8. J Biol Chem 2011;286:13282-91. [Crossref] [PubMed]
- Micheau O, Lens S, Gaide O, et al. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol 2001;21:5299-305. [Crossref] [PubMed]
- Ehlken H, Krishna-Subramanian S, Ochoa-Callejero L, et al. Death receptor-independent FADD signalling triggers hepatitis and hepatocellular carcinoma in mice with liver parenchymal cell-specific NEMO knockout. Cell Death Differ 2014;21:1721-32. [Crossref] [PubMed]
- Liedtke C, Bangen JM, Freimuth J, et al. Loss of caspase-8 protects mice against inflammation-related hepatocarcinogenesis but induces non-apoptotic liver injury. Gastroenterology 2011;141:2176-87. [Crossref] [PubMed]
- Shimizu Y, Peltzer N, Sevko A, et al. The linear ubiquitin chain assembly complex acts as a liver tumor suppressor and inhibits hepatocyte apoptosis and hepatitis. Hepatology 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Ashkenazi A, Salvesen G. Regulated cell death: signaling and mechanisms. Annu Rev Cell Dev Biol 2014;30:337-56. [Crossref] [PubMed]
- Estornes Y, Aguileta MA, Dubuisson C, et al. RIPK1 promotes death receptor-independent caspase-8-mediated apoptosis under unresolved ER stress conditions. Cell Death Dis 2014;5:e1555 [Crossref] [PubMed]
- Biton S, Ashkenazi A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-alpha feedforward signaling. Cell 2011;145:92-103. [Crossref] [PubMed]
- Yang Y, Xia F, Hermance N, et al. A cytosolic ATM/NEMO/RIP1 complex recruits TAK1 to mediate the NF-kappaB and p38 mitogen-activated protein kinase (MAPK)/MAPK-activated protein 2 responses to DNA damage. Mol Cell Biol 2011;31:2774-86. [Crossref] [PubMed]
- Tenev T, Bianchi K, Darding M, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell 2011;43:432-48. [Crossref] [PubMed]
- Feoktistova M, Geserick P, Kellert B, et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell 2011;43:449-63. [Crossref] [PubMed]
- Khurana S, Oberdoerffer P. Replication Stress: A Lifetime of Epigenetic Change. Genes (Basel) 2015;6:858-77. [Crossref] [PubMed]
- Dara L, Liu ZX, Kaplowitz N. Questions and controversies: the role of necroptosis in liver disease. Cell Death Discov 2016;2:16089. [Crossref] [PubMed]
- Maeda S, Kamata H, Luo JL, et al. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005;121:977-90. [Crossref] [PubMed]
- Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet 2015;47:505-11. [Crossref] [PubMed]
- Shibata T, Aburatani H. Exploration of liver cancer genomes. Nat Rev Gastroenterol Hepatol 2014;11:340-9. [Crossref] [PubMed]
- Aigelsreiter A, Haybaeck J, Schauer S, et al. NEMO expression in human hepatocellular carcinoma and its association with clinical outcome. Hum Pathol 2012;43:1012-9. [Crossref] [PubMed]


