
Targeting Bruton’s tyrosine kinase expression levels through microRNAs in chronic lymphocytic leukemia treatment
Introduction: role of Bruton’s tyrosine kinase (BTK) in chronic lymphocytic leukemia (CLL)
CLL is the most common leukemia in the western world and is characterized by the accumulation of monoclonal mature circulating CD5+ B cells that generally express low levels of surface immunoglobulin (Ig) (1,2). Many lines of evidence indicate that chronic signaling through the B-cell receptor (BCR) plays a key role in CLL pathogenesis (1,2). CLL prognosis is correlated with the BCR somatic hypermutation status and the CLL BCR repertoire is highly restricted. Often, CLL cells show constitutive activation of several kinases that are activated immediately downstream of the BCR. Thus, the BCR signaling pathway is aberrantly active in CLL and may play a role in disease development. One of the signal transduction molecules downstream of the BCR is BTK, a Tec family non-receptor kinase that is primarily expressed in most hematopoietic lineages, but not in T cells (3,4). BTK has been shown to be essential for several constitutively active pathways implicated in CLL cell survival, including the AKT, ERK and NF-κB pathway (2,3). Importantly, small molecule inhibitors of BTK have shown impressive anti-tumor activity in clinical studies, with high response rates in various B cell malignancies, in particular CLL (3).
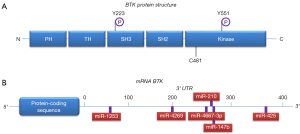
BTK consists of several domains implicated in interactions with other proteins, as well as a kinase domain that is crucial for its enzymatic function (Figure 1). Upon BCR engagement, BTK is phosphorylated by LYN or SYK in the kinase domain at position Y551, following recruitment to the cell membrane by phosphatidylinositol-3,4,5-trisphosphate (PIP3), which is generated by phosphatidylinositol 3-kinase (PI3K) (Figure 2) (3,4). When the BTK kinase domain is phosphorylated, it subsequently undergoes autophosphorylation in the Src homology (SH) 3 domain at position Y223 (Figure 1). Subsequently, BTK can activate and phosphorylate other signaling molecules including AKT and phospholipase Cγ2 (PLCγ2). This will lead to the activation of the mitogen-activated protein kinase (MAPK) signaling pathway and NF-κB translocation from the cytoplasm to the nucleus, which—together with AKT—is crucially involved in B cell survival, proliferation and differentiation (Figure 2).
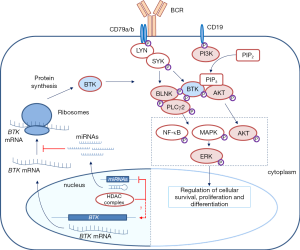
BTK activity likely represents a critical step in BCR signaling, as inferred from several findings showing that BTK protein levels are decisive for B cell function (4). First, sub-physiological protein expression of Btk cannot rescue the x-linked immunodeficiency (xid) phenotype in Btk-deficient mice, whereas physiological Btk levels can completely restore the xid phenotype in Btk-deficient mice (3). Upon BCR activation in mouse and human B cells, BTK protein levels are upregulated, although the mechanisms involved are not fully understood (6,7). It has been shown that BTK can induce its own transcription in an NF-κB-dependent fashion, involving an NF-κB binding site in the BTK promoter (8). In addition, microRNAs (miRNAs), including miR-185, play an important role in the post-translational regulation of BTK expression (9). Therefore, it is conceivable that not only BTK function but also BTK protein expression levels are very relevant for normal B cell function, indicating that BTK dysregulation could play an important role in B cell malignancies. This is in agreement with recent findings showing that increased expression of BTK is associated with autoimmune disease. Belver et al. showed that targeted deletion of Dicer in B cells in mice results in lower levels of miR-185, accumulation of BTK and an autoimmune phenotype (9). In addition, transgenic B-cell-specific BTK-overexpression leads to spontaneous development of autoimmunity (6). These BTK overexpressing mice do not spontaneously develop CLL, but BTK overexpression accelerated leukemogenesis in a CLL mouse model (10). Elevated BTK protein expression was also found in peripheral blood B cells from patients with systemic autoimmune disease (7). Thus, proper control of BTK protein levels is vital for B cell homeostasis, both in mice and in human.
Targeting BTK in CLL
Because BCR signaling was implicated in CLL pathogenesis and because of the critical role of BTK in BCR signaling, it became attractive to target BTK to develop new treatment modalities for B cell malignancies. Several BTK inhibitors were developed of which the irreversible inhibitor ibrutinib induced objective clinical responses in dogs with spontaneous B-cell non-Hodgkin lymphoma (3,11). Ibrutinib is an orally bioavailable, irreversible BTK inhibitor that binds specifically to a cysteine residue (C481) in the ATP-binding region of the BTK kinase domain (Figure 1) and thereby inhibits its enzymatic activity (11). Effective anti-tumor activity of ibrutinib was convincingly shown in clinical studies of relapsed/refractory CLL (12), rapidly leading to FDA approval of ibrutinib in November 2013. More recently, ibrutinib has also been approved for frontline use in CLL patients (13). Despite considerable clinical success of ibrutinib, there are some emerging concerns associated with its long-term clinical application. Ibrutinib is not a recommended choice for young patients: it commits patients to lifelong therapy, which can pose many issues, including lack of compliance and possible long-term toxicities. Moreover, continuous therapy was reported to lead to selection or outgrowth of resistant CLL clones, as described in a subset of CLL patients who showed disease relapse upon ibrutinib therapy. This phenomenon was attributed to either a BTK C481S mutation (the site of attack of ibrutinib), or to activating mutations in PLCγ2 (R665W, S707Y and L845F), an important downstream substrate of BTK (Figure 2) (14). The progression of disease while on ibrutinib therapy is associated with a poor outcome, as some patients experience rebound lymphadenopathy and symptoms upon discontinuation. This demands the need to develop novel therapeutic strategies, in particular the development of treatment combination strategies that could improve the current success rates without increasing the toxicities. In this regard, ibrutinib is currently being tested in combination with e.g., rituximab against FCR (fludarabine, cyclophosphamide and rituximab) in young CLL patients (NCT02048813) and against bendamustine/rituximab (BR) in CLL patients aged >65 years (NCT01886872).
Targeting BTK through miRNAs
In the article by Bottoni et al., a novel strategy to dually target both the kinase activity and the expression levels of BTK was explored (5). Essentially, they used inhibition of histone deacetylates (HDACs) to increase the expression of miRNAs controlling BTK expression. HDACs function in repressor complexes to promote chromatin compaction by regulating deacetylation and demethylation of lysine residues on histones, thus resulting in epigenetic gene silencing. HDACs have previously been shown to silence the expression of various miRNAs implicated in CLL pathogenesis, including miR-15a, miR-16 and miR-29b (15), known to be epigenetically regulated in CLL. However, the role of miRNAs in regulating BTK expression in the context of CLL and the effects of HDACs on BTK-targeting miRNAs remained unexplored.
miRNAs are a class of endogenous noncoding double-stranded RNA molecules of about 19–23 nucleotides in length, which regulate gene expression at either the transcriptional or the post-transcriptional level (16). Using their guide strand, called the miRNA-induced silencing complex (miRISC), miRNAs bind to miRNA recognition elements (MREs) within the 3' or 5' untranslated regions (UTRs) of target messenger RNA (mRNA) molecules and thereby block mRNA translation, reduce mRNA stability or induce mRNA cleavage (16). miRNAs are critical for numerous aspects of the regulation and maintenance of the mammalian immune system (17). Regarding CLL, it has been reported that about 50% of patients have deletions at 13q14, which are associated with downregulation of miR-15a and miR-16 (18). Loss of this miRNA cluster results in overexpression of survival genes, including BCL-2 and MCL-1, thereby inducing resistance to apoptosis. In addition, several other miRNAs have been implicated in the regulation of the expression of proteins with oncogenic and tumor suppressor function in CLL [recently reviewed in (19)]. Bottoni et al. demonstrated that a set of six miRNAs predicted to target BTK with high specificity by several algorithms (miR-147b, miR-210, miR-425, miR-1253, miR-4269, and miR-4667-3p) substantially decreased BTK expression in Mec2 cell lines derived from CLL patients (5). Putative binding sites for these miRNAs were identified within the BTK 3' UTR, and evidence was provided for direct interaction (Figure 1). Most interestingly, they found that ectopic expression of two of these miRNAs, miR-210 and miR-425, significantly reduced expression of BTK protein across the primary CLL samples tested. Conversely, antagonizing miR-210 and miR-425 activity resulted in increased BTK protein levels in Mec2 cells. The finding that the levels of several BTK-targeting miRNAs, including miR-210 and miR-425, were significantly lower in CLL cells than in normal B cells provided a mechanistic explanation for the robust expression of BTK in CLL (5).
Combination of ibrutinib and HDAC inhibitors as a novel therapeutic modality
Next, Bottoni et al. hypothesized that HDAC repressor complexes could mediate silencing of BTK-targeting miRNAs in CLL, as was previously shown for other sets of miRNAs by the same group (15). Indeed, they established that HDAC repressor complexes were recruited specifically to the promoter regions of the BTK-targeting miRNAs in CLL. Treatment of primary CLL samples either with the HDAC inhibitors (HDACi) panobinostat and abexinostat or small interfering RNA-mediated knockdown of HDAC resulted in increased expression of BTK-targeting miRNAs and as a consequence decreased BTK mRNA and protein expression (5). The downregulation of the BTK-targeting miRNAs and response to HDACi was independent of cytogenetic group in CLL.
Since ibrutinib has already shown high success rates in the clinical management of CLL, the authors sought to combine ibrutinib with HDACi to target both the kinase activity and protein expression levels of BTK, using two entirely different molecular mechanisms. Whereas ibrutinib treatment inhibited BTK phosphorylation (while preserving its expression levels), abexinostat treatment resulted in significant reduction of total BTK protein and thereby also of phosphorylated-BTK. This reduction in activating status was also seen in distal downstream effectors of BTK, such as phosphorylated PLCγ2, p-ERK and p-AKT, indicating reduced BCR/BTK-mediated survival signaling. More importantly, the combination of HDACi with ibrutinib induced synergistic cytotoxicity in primary CLL cells compared to either agent alone. The effect of ibrutinib and HDACi combination on abrogating BTK-mediated signaling and diminishing CLL cell survival were also observed in the widely studied EµTCL-1 mouse model of CLL (20) and was superior to either agent alone. Adoptive transfer experiments demonstrated that combination of abexinostat and ibrutinib in vivo efficiently reduced leukemic cell counts (5). Although not discussed, it would be very interesting to establish whether HDACi and ibrutinib have a synergistic effect on survival of EµTCL-1 mice.
Similar to other cancer treatments, selection or outgrowth of resistant clones is a major problem in clinical management of CLL. This also holds true both for conventional chemotherapeutics and novel targeted drugs including ibrutinib, which pose considerable selection pressure for the CLL cells to escape elimination. Therefore, Bottoni et al. also investigated the outcome of HDACi on ibrutinib-resistant CLL cells that harbor the C481S BTK mutation (5,14). The authors tested the effects of HDACi on CLL before ibrutinib therapy and after the acquisition of the C481S BTK mutation. Depletion of BTK protein and reduced levels of p-PLCγ2, p-ERK and p-AKT were observed, regardless of the BTK C481S mutational status or response to ibrutinib therapy.
In various forms of cancer HDACi is a successful therapy leading to amelioration of disease. Given the general effects of HDACi on gene expression, it is remarkable that Bottoni et al. found that HDACi specifically increased BTK-targeting miRNAs. In their studies, abexinostat treatment reduced BTK expression, but did not appear to affect other signal transduction molecules downstream of the BCR, such as PLCγ2, AKT and ERK. It has been shown that several miRNAs, including miR-155, are overexpressed in CLL, diffuse large B cell lymphoma (DLBCL) and multiple myeloma (MM) patients (21). Because the inositol phosphatase SHIP1 is a primary target of miR-155 (22), HDACi may lead to lower expression of SHIP1, which negatively regulates BCR signaling by dephosphorylating PIP3. Thus, it is conceivable that via miR-155 and SHIP1, the effectivity of BTK-targeting therapy mediated by HDACi would be dampened. Given the observed significant downregulation of PLCγ2 phosphorylation, however, there is no evidence for decreased SHIP1 activity. Bottoni et al. did not investigate direct effects of HDACi on BTK locus accessibility, e.g., through promoter or enhancer elements in the BTK locus. However, the observed robust downregulation of BTK protein levels by HDACi clearly indicates that the influence of such direct effects on BTK expression is negligible.
Future perspectives
Ibrutinib treatment has shown clear clinical anti-tumor effects in CLL and various other B cell malignancies (3,12,13), even though this compound is not highly specific: it also binds to EGFR, TEC and ITK (11), potentially compromising its therapeutic index. It remains unclear what the physiological effects would be of putative inhibition of TEC activity by ibrutinib in CLL cells. On the one hand it has been reported in mice that Tec can partly compensate for the absence of Btk in B cells, but on the other hand we recently found that Akt signaling was increased in Tec-deficient B cells (23). Ibrutinib treatment might enhance immune responses or autoimmune symptoms in patients, as it has been found that by targeting ITK in T cells ibrutinib drives differentiation of T helper cells to a Th1 phenotype (24). Nevertheless, it is not very likely that the effectiveness of ibrutinib is partially due to its non-specific nature, because the more selective BTK inhibitor acalabrutinib has also shown high effectiveness in relapsed CLL in clinical trials (25). It is attractive to explore combination therapies of HDACi with acalabrutinib, which has a promising safety profile, or other selective BTK inhibitors. Remarkably, the exact mechanism how ibrutinib ameliorates CLL disease is currently unknown. It is believed that BTK inhibition has a direct effect on B cell survival, but there is also evidence that it has major effects on B cell migration and adhesion and thereby on B cell homing and retention in a favorable micro-environment (2,3). It would thus be interesting to test the effects of HDACi (in combination with ibrutinib or acalabrutinib) on CLL cell migration and adhesion.
Next to combining HDACi with ibrutinib, combinations with a BCL-2 inhibitor or inhibitors that target other BCR signaling molecules, such as PI3K or SYK, would be very interesting with respect to treatment of CLL or other B cell malignancies. Furthermore, it would be worthwhile to develop compounds that efficiently and selectively target PLCγ2, because of the ibrutinib-induced gain-of-function mutations in PLCγ2 (14). Although CLL cells with C481S-mutant BTK can be very well targeted by HDACi, patients with an ibrutinib-induced constitutive active PLCγ2 mutation will not benefit from HDACi because it does not affect PLCγ2 protein expression.
The findings by Bottoni et al. (5) provide convincing evidence that effective reduction of BTK expression by HDAC inhibitors and targeting enzymatic activity of BTK may be a promising therapeutic modality that suppresses survival signals in CLL. This raises an important question: should ibrutinib and HDACi be given simulatenously, or should HDACi be started before ibrutinib treatment or after ibrutinib treatment. Because of current problems of toxicity in CLL patients with HDACi and side-effects from ibrutinib, it might not be wise to give the therapeutics simultaneously. The rationale of giving HDACi as second-line therapy after ibrutinib may be more sensible, since the authors showed that C481S-mutant BTK can still be targeted. Additional studies are required to establish the best treatment strategy and, importantly, should show whether HDACi is beneficial for patients that show a limited response to BTK inhibition. Furthermore, other leukemic diseases, such as MM, mantle cell leukemia, and activated B-cell-like DLBCL, could very well benefit from combination therapy with ibrutinib and HDACi. Finally, this might also be the case for patients with autoimmune diseases, such as rheumatoid arthritis (RA). Currently, clinical trials with BTK inhibition are ongoing and BTK was recently shown to be specifically enhanced in RA patients expressing anti-citrullinated protein antibodies (7). In this context, there is also a rationale for HDACi treatment: in follicular B cells, miR-185 expression downregulates BCR responsiveness by BTK-dependent mechanisms and absence of miR-185 leads to the development of systemic autoimmune disease (9).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Wei Xu (Division of Respiratory Disease, Department of Geriatrics, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.04.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med 2005;352:804-15. [Crossref] [PubMed]
- Muggen AF, Singh SP, Hendriks RW, et al. Targeting Signaling Pathways in Chronic Lymphocytic Leukemia. Curr Cancer Drug Targets 2016;16:669-88. [Crossref] [PubMed]
- Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton's tyrosine kinase in B cell malignancies. Nat Rev Cancer 2014;14:219-32. [Crossref] [PubMed]
- Corneth OB, Klein Wolterink RG, Hendriks RW. BTK Signaling in B Cell Differentiation and Autoimmunity. Curr Top Microbiol Immunol 2016;393:67-105. [Crossref] [PubMed]
- Bottoni A, Rizzotto L, Lai TH, et al. Targeting BTK through microRNA in chronic lymphocytic leukemia. Blood 2016;128:3101-12. [PubMed]
- Kil LP, de Bruijn MJ, van Nimwegen M, et al. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood 2012;119:3744-56. [Crossref] [PubMed]
- Corneth OB, Verstappen GM, Paulissen SM, et al. Enhanced Bruton's tyrosine kinase activity in peripheral blood B lymphocytes of autoimmune disease patients. Arthritis Rheumatol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Yu L, Mohamed AJ, Simonson OE, et al. Proteasome-dependent autoregulation of Bruton tyrosine kinase (Btk) promoter via NF-kappaB. Blood 2008;111:4617-26. [Crossref] [PubMed]
- Belver L, de Yebenes VG, Ramiro AR. MicroRNAs prevent the generation of autoreactive antibodies. Immunity 2010;33:713-22. [Crossref] [PubMed]
- Kil LP, de Bruijn MJ, van Hulst JA, et al. Bruton's tyrosine kinase mediated signaling enhances leukemogenesis in a mouse model for chronic lymphocytic leukemia. Am J Blood Res 2013;3:71-83. [PubMed]
- Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A 2010;107:13075-80. [Crossref] [PubMed]
- Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369:32-42. [Crossref] [PubMed]
- Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med 2015;373:2425-37. [Crossref] [PubMed]
- Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med 2014;370:2286-94. [Crossref] [PubMed]
- Sampath D, Liu C, Vasan K, et al. Histone deacetylases mediate the silencing of miR-15a, miR-16, and miR-29b in chronic lymphocytic leukemia. Blood 2012;119:1162-72. [Crossref] [PubMed]
- Lindsay MA. microRNAs and the immune response. Trends Immunol 2008;29:343-51. [Crossref] [PubMed]
- Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell 2009;136:26-36. [Crossref] [PubMed]
- Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 2002;99:15524-9. [Crossref] [PubMed]
- Balatti V, Acunzo M, Pekarky Y, et al. Novel mechanisms of regulation of miRNAs in CLL. Trends Cancer 2016;2:134-43. [Crossref] [PubMed]
- Bichi R, Shinton SA, Martin ES, et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci U S A 2002;99:6955-60. [Crossref] [PubMed]
- Eis PS, Tam W, Sun L, et al. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci U S A 2005;102:3627-32. [Crossref] [PubMed]
- O'Connell RM, Chaudhuri AA, Rao DS, et al. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci U S A 2009;106:7113-8. [Crossref] [PubMed]
- de Bruijn MJ, Rip J, van der Ploeg EK, et al. Distinct and Overlapping Functions of TEC Kinase and BTK in B Cell Receptor Signaling. J Immunol 2017;198:3058-68. [Crossref] [PubMed]
- Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013;122:2539-49. [Crossref] [PubMed]
- Byrd JC, Harrington B, O'Brien S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 2016;374:323-32. [Crossref] [PubMed]


