
Intestinal stem cell injury and protection during cancer therapy
Introduction
Radiation and chemotherapy remain the most effective and widely used cancer treatments. Despite improvements in the delivery, dosing or combination of treatments, significant toxicities to normal tissues remain. Approximately 70% of all cancer patients receive radiation therapy during their care, which plays a critical role in 25% of all cancer cures (1,2). There are currently more than 10 million cancer survivors in the United States, necessitating measures to reduce treatment-related side effects. A better understanding of the molecular and cellular basis of these treatments, the related side effects, and new interventions can help ameliorate or prevent short-term and long-term toxicities of cancer therapies (1-3).
Most patients undergoing radiation to the abdomen, pelvis, or rectum will develop acute enteritis, which is dose limiting, while 5% to 15% of them will develop chronic problems (3,4). Radiation enteropathy is classified as early (acute) or delayed (chronic) (3). Early radiation enteropathy occurs during or shortly after radiotherapy, characterized by the death of rapidly proliferating crypt cells, resulting in epithelial barrier breakdown and inflammation (radiation mucositis). Delayed radiation enteropathy occurs months or later after radiotherapy, characterized by intestinal dysfunction associated with vascular sclerosis and progressive intestinal wall fibrosis, a process involving a complex interplay of various cell types, factors, and extracellular matrix (3). Loss of intestinal stem and progenitor cells plays a key role in acute radiation side effects in abdominal radiotherapy (2), which has attracted great interests in radioprotective drugs (1,2).
Intestinal stem cells (ISCs)
The single-layer columnar epithelium of the small intestine is one of the most rapidly renewing tissues in an adult mammal with a renewal cycle estimated to be 3-5 days in mice. The intense proliferation that fuels this self renewal process is confined to the crypts (5-7). Differentiated epithelial cells such as absorptive enterocytes, mucus-secreting goblet cells, enteroendocrine cells, and Paneth cells are generally well-defined by morphology and markers (8-10). Deep crypt secretory cells (11) may represent the colon counterparts of Paneth cells. However, the precise location and characteristics of ISCs remained elusive for a long time. Studies in the 1970s and 1980s defined two major populations of ISCs based on their locations: the stem cell zone model proposed by Cheng and Leblond defined the crypt base columnar cells (CBCs) sandwiched between Paneth cells (6,8), and the +4 label retaining cells (LRC) proposed by Potten and colleagues appeared radiosensitive (12).
Identification of ISC markers and generation of reporter mice have led to an explosion of ISC studies in the last five years (10). Most adult stem cell niches are coinhabited by cycling and quiescent stem cells. In the intestine, lineage tracing experiments revealed that Lgr5(+) (13) cells are frequently cycling stem cells, while Bmi1(+) (14), mTert(+) (15), Hopx(+) (16) and Lrig1(+) (17,18) cells appear more quiescent (10). Additional ISC-enriched populations are marked by CD133 (19,20), Musashi-1 (21), or dye-exclusion (side population, SP) (22) without definitive lineage tracing data. Since the expression of some reporters does not always recapitulate that of endogenous stem cell markers (Table 1), efforts are underway to use cell surface markers for ISC isolation (35,36).
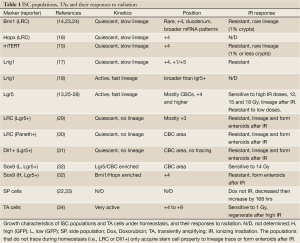
Full table
Another breakthrough in the ISC field is the successful culture of isolated crypts and ISCs (37) in so called enteroids (38) or organoids assays. Tracing experiments indicated that the Lgr5(+) stem-cell hierarchy is maintained in enteroids containing four major differentiated epithelial lineages. Since then, similar approaches have been used to culture Lgr5+ cells, crypts, or marker enriched cells from mouse and human GI tract (10). This “in vitro” clonogenic assay is expected to greatly help the understanding of stem cell injury and regeneration regulated by cell autonomous mechanisms, and certainly can be adapted to include niche components as discussed later.
Intestinal response to radiation and chemotherapy in mice
Radiation and chemotherapy cause DNA damage and selectively target rapidly proliferating cells such as cancer cells and normal cells that undergo rapid self renewal, including those in the gastrointestinal (GI) tract. The response of the small intestine to ionizing radiation (IR) has been well characterized in mice, and damage to the clonogenic cells in the crypts through apoptosis plays an important role in IR-induced acute GI damage (34). Radiation at 8 Gy or lower doses causes obvious apoptosis in the crypts, and subsequent shortening of villi over a period of 5-7 days. This is followed by full recovery, suggesting little or no permanent injury to the stem cell compartment. After receiving greater than 14 Gy of total body irradiation (TBI), mice die between 7 and 12 days due to damage to the small intestine and complications known as GI syndrome, which cannot be rescued by bone marrow (BM) transplantation (34,39,40), or by any approved treatment. This higher dose causes complete sterilization of most crypts and severe loss of the epithelium, accompanied by a powerful regenerative response measured using a micro colony assay 3-4 days post IR (41,42). This so-called clonal regeneration process with characteristic regenerated crypts is widely used as the “in vivo” clonogenic assay. Therefore, apoptosis, cell proliferation, micro colony assays, and animal survival following 14 Gy (or higher) TBI or subtotal body irradiation have traditionally been used to assess ISC injury (34).
Radiation responses of several ISC populations have recently been examined in mice (Table 1). High doses of radiation (12 Gy or higher) cause loss of CBCs (23,25,26), and activation of quiescent stem cells (10,33) (Figure 1). Some early progenitors can revert back to stem cells only after IR, showing more efficient lineage tracing and formation of enteroids. These include some label retaining cells (LRC) (29), delta ligand expressing Dll1+ cells (31), and Sox9-GFP high cells (32), all expressing Lgr 5 in the +4 region (+3-+7), where transiently amplifying (TA) cells also reside. LRC/Paneth cells also appear to enter cell cycle after IR (30). The chemotherapeutic agent doxorubicin (Adriamycin) caused increased SP before crypt regeneration (22,33). With in-depth gene expression analysis (27) and single cell transcripts analysis (24), a picture is emerging that ISC populations and early progenitors are plastic, overlap in gene expression profiles, and can interconvert upon injury or genetic ablation, while fully recovered crypts resume the CBC/Paneth cell pattern at the crypt base (Figure 1) (10). The relative contribution of these populations to regeneration, their overlap, activation mechanisms, and the effects of radiation on marker expression remain to be determined.
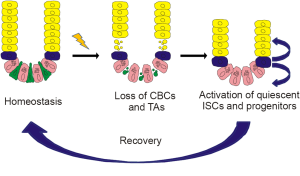
Pathways controlling GI and ISC injury in response to genotoxic stress
IR-induced acute GI injury is characterized by a rapid loss of stem cells, breach in barrier function and lethality, in a time frame coinciding with the 5-day renewal cycle. Current understanding of DNA damage-induced intestinal injury came largely from genetically manipulated mice. Radiation-induced stem cell killing is controlled by p53-dependent early apoptosis and late mitotic death suppressed by p53. Regulators in DNA damage sensing, replication and repair, checkpoint functions and apoptosis significantly impact crypt radiosensitivity, consistent with generally cell autonomous mechanisms of DNA damage response as defined in humans, mice and other model organisms (43,44).
p53—a paradoxical role in IR-induced GI injury
p53 is one of the most important proteins protecting against carcinogenesis in mammals, and yet its activation by severe genotoxic stress leads to p53-dependent pathologies (45). Upon activation, p53 engages transcriptional programs to initiate apoptosis and cell cycle arrest, with opposing roles in cell survival (45-47). The BH3-only protein PUMA (48) and cyclin-dependent kinase (CDK) inhibitor p21 (49) are two major p53 effectors (Figure 2). p53 activation and responses are highly tissue-specific, likely reflecting selective activation of downstream p53 targets (45,46). Radiosensitive tissues tend to have high levels of p53 activity (50,51) and induction of apoptosis and apoptotic targets (52,53), while radioresistance tissues show selective induction of cell cycle regulators with little or no apoptosis (54,55). Loss of p53 protects the hematopoietic (HP) system and skin against IR and chemotherapy-induced injuries (56,57), and the small intestine from chemotherapy-induced apoptosis and mucositis (58,59) by blocking apoptosis. However, loss of p53 unexpectedly exacerbates GI damage and accelerated GI syndrome (39,60) despite blocked apoptosis (60). Moreover, the delayed mitotic cell death in the crypts occurring 24 hours or later after IR is exacerbated by p53 loss (61).
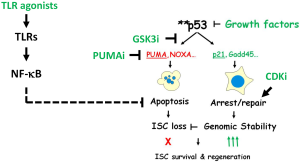
PUMA and p21—the battle of killing and mending
The answer to the paradoxical role of p53 came from genetically uncoupling of two arms of p53 responses using mice that deficient in PUMA, p21 or both (PUMA/p21) (62). The induction of PUMA and p21 by IR was mostly p53-dependent in the GI epithelium. In PUMA knockout (KO) mice, the early apoptosis was blocked, leading to increased ISC survival and regeneration, animal survival after high dose irradiation (25). A strong protection was observed in the CBCs besides the +4 region (25,28). In p21 KO mice, cell cycle arrest and DNA repair was lost, leading to shortened survival, accelerated crypt regeneration associated with massive nonapoptotic cell death, aberrant cell-cycle progression, persistent DNA damage, rampant replication stress, and chromosomal instability. Lack of p21 induction in p53 KO mice, or in PUMA/p21 double knockout (DKO) mice drastically elevated the delayed mitotic death, which was most pronounced during crypt regeneration despite blocked early apoptosis (Figure 2) (62). Loss of p21 also led to reduced cell viability after DNA damage (46), and abolished GI protection by “super p53” (63,64) and HP protection by CDK4/6 inhibition after IR (65). PUMA deficiency strongly protected against IR-induced hematopoietic stem cell apoptosis and lethality (66-71), which might also require p21. It would be interesting to see if blocking PUMA-dependent apoptosis potentiates p21 or p53-induced stem cell protection.
Bcl-2 family
The Bcl-2 family is a group of evolutionarily conserved regulators of apoptosis induced by diverse stimuli (72,73), and executes p53-dependent apoptosis through the mitochondrial pathway following severe genotoxic stress (23,46,74). This family is further divided into three subfamilies based on their functions and structures: antiapoptotic Bcl-2 like proteins, Bax-like proapoptotic members, and the BH3-only proapoptotic members such as PUMA and Noxa. The BH3-only proteins are responsible for sensing and transmitting apoptotic signals to other Bcl-2 family members (74). Mice deficient in NOXA (75), also a p53 target, BAX or BAK (25,76), or BAX and BAK in the GI epithelium (63) were resistance to IR-induced crypt apoptosis, but BAX or BAK appear to mediate crypt apoptosis and survival only at GI-toxic doses, unlike their largely overlapping functions in development (77). BCL-2 KO (78) or BCL-w KO (79) mice showed increased apoptosis with 5-fluorouracil (5-FU) treatment or IR in the small intestinal crypts (80). In contrast, the Bcl-2 family plays little or no role in spontaneous crypt apoptosis (81).
DNA repair proteins
Deficiency in DNA repair proteins generally elevates intestinal radiosensitivity. ATM (ataxia telangiectasia mutated) KO mice showed accelerated GI-injury and lethality (82). Knockout of 53BP1 (83), or poly ADP-ribosepolymerase-1 (PARP-1) (84), led to decreased crypt survival after treatment with alkylating agents or IR. Loss of RAD50, MRE11, or DNAPK reduced crypt survival, while enhanced Rad50 response engaged p53-dependent protection (85). These data suggest that DNA repair protects against radiation-induced stem cell loss, without affecting early apoptosis or cell cycle arrest (83). Nonapoptotic killing of ISCs due to failed DNA repair likely involve replication stress, persistence DNA damage and chromosomal instability, as found in p21 KO mice (62).
Mismatch repair (MMR) proteins
Mutations in MMR proteins are found in the hereditary nonpolyposis colorectal cancer (HNPCC) syndrome (86). In addition to repair of mismatch DNA lesions, MMR proteins appear to interact with the p53 pathway to engage apoptosis depending on the type of DNA lesions (87). Deletion of MSH2 (88), MLH1, or PMS2 (89,90) led to resistance to apoptosis induced by IR or chemotherapy [5-FU, cisplatin, temozolomide, and N-methyl-N'-nitro-N-nitrosoguanidine (MNNG)]. In these studies, MMR deficiency enhanced carcinogenesis likely by elevating stem cell survival and mutation rates, where MNNG did not cause significant ISC loss or GI injury.
ISC survival measured by micro colony assay is not always correlated with apoptosis or animal survival in mice deficient in p21, p53 or MSH2 (62,91). Inability to assess ISC apoptosis and the “quality” of regenerated crypts might help explain why it was only recently discovered that some Lgr5+ and +4 cells, opposed to transiently amplifying cells (TA cells), are resistant to IR up to 8 Gy (Table 1) (26,28) (JY unpublished data), and the p53/p21 axis suppresses mitotic death and accelerated crypt regeneration independent of apoptosis (62). Therefore, parameters such as non-apoptotic cell death, the timing of crypt regeneration, DNA damage, and villus length should be considered to better measure ISC responses to IR.
Nuclear factor kappa B (NF-κB)
NF-κB regulates a wide variety of cellular functions, such as survival, proliferation, migration, and immune response, and its persistent activation leads to inflammation and cancer (92). TBI activated NF-κB/RelA heterodimers (93). NF-κB p50 KO mice showed elevated crypt apoptosis and sensitivity to IR-induced lethality and decreased crypt survival (93). Intestinal deletion of NF-κB/p65 activator IKKbeta led to increased epithelial apoptosis without treatment (94), suggesting transient NF-κB activation might improve ISC survival.
Prostaglandins (PGs) and Cox1 and Cox2
Prostaglandins are lipid second messengers that regulate intestinal epithelial apoptosis and proliferation, as well as immune responses. PGs are synthesized from arachidonic acid by either cyclooxygenase-1 (Cox-1) or cyclooxygenase-2 (Cox-2) (95). In particular, PGE2 shows strong radioprotective effects on the epithelium largely via the prostaglandin E2 receptor (EP2) (95). PGE2 suppressed IR-induced crypt apoptosis and enhanced crypt regeneration. PGE2 neutralizing antibody, COX1 KO, nonselective Cox inhibitors, but not COX2 KO, had opposite effects (96,97). These data suggest an important role of Cox1-mediated PGE2 production in the survival of irradiated ISCs.
Circadian clock
The circadian clock is an evolutionarily conserved intrinsic timekeeping mechanism that controls daily variations in multiple biological processes (98). It has been known that intestinal proliferation, migration, and radiation response follow a circadian rhythm (12). Recent work showed that clock components regulate ISC (99) and hair follicle regeneration (100) in flies by coordinating cell cycle progression, stem cell division and gene expression (99). Interestingly, sensitivity to chemotherapy was also regulated by circadian rhythm (101), which can be suppressed by selenium in mice (102). These findings suggest novel ways for stem cell protection.
Regulation of radiation-induced ISC injury and regeneration by the “niche” and beyond
Stem cell function is controlled by extracellular cues from the niche in addition to intrinsic programs. The stem cell niche refers to cellular components and various signals found in their surrounding microenvironment, which collectively play a key role in stem cell self renewal and quiescence (103,104). Non-epithelial cells and soluble mediators can modulate the survival, differentiation or proliferation of ISCs and progenitors during injury. These include the BM (40), endothelial cells, mesenchymal cells, enteric neurons (104,105), immune cells (106), and growth factors or other ligands (107). As discussed below, understanding the effects of non-epithelial cells or factors are important for intestinal protection against cancer treatments.
Vascular endothelial cells
The concept that GI and crypt radiosensitivity is determined by vascular endothelial cells was primarily based on the antiapoptotic effects of bFGF, acid sphingomyelinase (also called ceramide synthase, ASMase) gene KO (108) or anti-ceramide antibody (109) in endothelial cells. Vascular endothelial dysfunction is involved in pathogenesis of early and delayed radiation enteropathy (110). However, several key findings argue against a significant role of endothelial cell loss in IR-induced acute apoptosis of ISCs occurring within hours. FGFR was expressed in ISCs not just endothelial cells (111). Growth factors and ASMase KO block IR-induced and p53-dependent apoptosis in ISCs (28) or lymphoid tissues (112). ISC protection occurred with minimal change in endothelial cells (25); and high dose IR caused “target” switching” to epithelial cells (82). All suggest a direct effect of these conditions on ISC apoptosis independent of those in endothelial cells. Using mice reconstituted with ASMase KO BM, crypt culture, or tissue specific KO should provide a more definitive answer.
Inflammation and immune cells
Inflammation plays an important role in radiation-induced injury, though the cellular and molecular targets are likely complex and remain poorly understood (106). GI epithelium interacts with a plethora of commensal and foreign antigens, making the gut mucosa a strong responsive organ in radiation-induced inflammation (106). The relatively poor therapeutic efficiency of “classic” anti-inflammatory strategies compared with the pathway agents discussed would suggest that ISC and epithelial injury is likely the trigger of lethal inflammatory responses. Production of inflammatory cytokines and immune cell infiltration negatively impacts ISCs further in the delayed phase via the niche and systemic effects (113-115). Therefore, opportunities might exist for exploiting the immune system to repair and heal gut epithelium with sufficient protection of ISCs in the acute phase.
Intestinal protection and mechanisms
Radiation protectors are agents that reduce normal tissue damage when administered before or at the time of radiation for effectiveness. Mitigators do so when administered even after radiation exposure. The major classes discussed below include antioxidents, growth factors, TRL ligands, apoptosis and cell cycle targeted agents and cell-based therapies. Almost all these agents suppress radiation-induced apoptosis and are more effective given before radiation, reinforcing the importance of ISC loss in the acute radiation injury. Some also protect against chemotherapy-induced GI injury, while a few promote ISC regeneration and suppress inflammation. Most of these agents are pleiotropic and act on the intestinal epithelium and other cell types (i.e., HP, immune and endothelial cells), making defining precise cellular targets difficult. Many new agents are in development and have shown promises in preclinical testing (1). On the other hand, stem cell protection might increase cancer risk upon expansion of damaged ISCs (116,117), a possibility which should be carefully assessed.
Antioxidants and natural products
Reactive oxygen species (ROS) are generated by IR and can directly damage DNA and other macromolecules, or cause damage indirectly by depleting cellular antioxidants (2). Various antioxidants, including plant-derived phytochemicals, and superoxide dismutase (SOD) gene therapy protect normal tissue from radiation-induced mild tissue injury, but have limited activities for IR-induced severe GI damage (118). The use of some antioxidants during radiotherapy was associated with poorer tumor control in human trials (1). Interestingly, PHY906, a four-herb Chinese medicine formula, reduced chemo-induced GI injury in clinical trials and mice without affecting tumor responses (119). The effects were associated with enhanced stem cell regeneration and Wnt signaling (119). However, the selectivity of PHY906 and other agents in this class in normal cells is generally not understood. Amifostine is the only approved radiation protector in clinical use that can reduce toxicity in the lung and hematopoietic system after chemotherapy or radiation (1).
Growth factors
A wide variety of growth factors and cytokines protect mice against radiation-induced GI injury and apoptosis, and improve crypt survival when administered before or shortly after IR (107). These include fibroblast growth factor 1(FGF-1) (120), basic fibroblast growth factor (bFGF or FGF-2) and related peptides, insulin-like growth factor 1 (IGF-1) (28,108,121,122), IGF transgenic (123), keratinocyte growth factor (KGF), transforming growth factor beta 3 (TGFβ3), interleukin 11 (107), antagonist of transforming growth factor beta 2 receptor (TGFβR2) (124), glucagon-like peptide-2 (GLP-2) (125), Lgr5 ligand R-Spondin 1 (126), and stem cell factor (SCF) (127,128). However, epidermal growth factor (EGF) was ineffective (129). KGF (130) and R-Spondin 1 also protected against chemotherapy-induced GI-injury and apoptosis, which is p53-dependent (58,59). Recent mechanistic studies indicate that IGF-1 and bFGF suppress p53-mediated PUMA expression and apoptosis in ISCs through the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/MDM2 axis (28), perhaps a common mechanism underlying growth factor-mediated ISC protection. Palifermin, a recombinant human KGF, is approved for clinical use to reduce severe oral mucositis in cancer patients after myeloablative therapy with BM transplantation.
TLR ligands
Pattern recognition receptors (PRRs) are proteins expressed on the surface of cells of the innate immune system that specifically recognize pathogen-associated molecular patterns (PAMPs) from microbial pathogens or cellular stress, and damage-associated molecular patterns (DAMPs) from cell components released during cell damage. They are also expressed on intestinal epithelial cells and are important in GI injury (131,132). Commensal bacteria activate Toll-like receptors (TLRs) and a variety of responses in different cells (131,132). Simulating TLR signaling by TLR4 ligand lipopolysaccharide (LPS) (133), TLR5 ligand Flagellin (134) and derivative CBLB502 (135), or TLR9 agonist (136), protects intestinal epithelium via NF-κB activation and apoptosis suppression (Figure 2). LPS or Flagellin are too proinflammatory to be useful radiation protectors. In contrast, CBLB502 suppressed “cytokine storm” and apoptosis in epithelial and endothelial cells, and effectively protected mice and rhesus monkeys against lethal TBI, given before or shortly after IR (135). Whether these agents engage p53-dependent responses in ISCs was unclear. Uncoupling NF-κB’s prosurvival and proinflammatory activities, much like the p53 pathway, might help find more effective radiation protectors.
Apoptosis and cell cycle targeted agents
A better understanding of p53 function in radiation-induced intestinal injury has an important implication. Unlike in the HP system, temporary suppression of p53 is predicted to be detrimental to the irradiated GI tract and ISCs. The differences between the HP and GI systems might reflect a significant difference in DNA repair in respective stem or progenitor compartments and is worth exploring (as discussed before). PUMA deficiency or p21 elevation did not significantly predispose mice to spontaneous carcinogenesis or aging (46,47). Small molecule PUMA inhibitors (137) and CDK inhibitors (69) are currently in development for radiation protection. Glycogen synthase kinase 3 (GSK3β) inhibitors might be another option by selectively blocking p53-dependent apoptosis (138). Growth factors might also suppress GSK3 via activation of PI3K/AKT (139).
Bone marrow-derived cells
Transplantation of large numbers of BM-derived cells protected against IR-induced GI injury, suppressed apoptosis and improved crypt and whole animal survival in mice. These include human mesenchymal stem cells (140,141), BM stromal cells (142), or whole marrow (143). BM-derived cells can give rise to several cell types outside of the hematopoietic system (144-146). However, even in the setting of GI injury, BM-derived cells were found to be incorporated into the human or mouse gastrointestinal tract at very low frequencies (147-149), excluding a direct role in tissue repair. Instead, paracrine signaling is likely to be important due to a systemic increase of growth factors such as bFGF, platelet-derived growth factor (PDGF), KGF and R-Spondin1 (142,143). The hematopoietic (CD45+) populations did not appear to play an important role in this process (142).
Conclusions
Successful cancer treatment by radiation and chemotherapy relies on selective killing of cancer cells with adequate protection of the normal tissue (133). Radiation and chemotherapy-induced GI injury is complex. In radiation-induced acute GI injury, both apoptotic and nonapoptotic cell death of ISCs play important roles and are largely regulated by cell-autonomous mechanisms. The epithelium and ISC injury in the delayed phase is less understood and involves additional cell types and the immune system. Understanding the differences between normal and tumor cells’ responses to genotoxic stress can lead to new rational approaches for selective protection of normal cells, such as suppression of p53-dependent apoptosis, enhanced DNA repair, activation of NF-κB, induced stem cell quiescence, and suppression of inflammation. Many challenges as well as opportunities lie ahead for GI protection against severe genotoxic stress. These will include the identification of additional regulators of ISC survival, better defining the role of non-epithelial cells and factors, and development of selective protectors. Crypt and ISC culture might prove to be a very useful model for many of these studies by enabling manipulation of pathways, validation of human relevance, and a high throughput platform for drug discovery. Lastly, short-term benefits of normal tissue and stem cell protection, and long-term risk of organ failure due to stem exhaustion or carcinogenesis due to damaged stem cells, need to be balanced.
Acknowledgments
The author would like to thank lab members for critical reading and discussion.
Funding: The work in author’s laboratory and this review is supported in part by NIH grants U01-DK085570, 1R01CA129829-01A1, American Cancer Society grant RGS-10-124-01-CCE (J Yu). The Yu laboratory is a member of the Intestinal Stem Cell Consortium, supported by NIDDK and NIAID (U01).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Daohong Zhou and Chuan-Yuan Li) for the series “Stem Cells in Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2013.07.03). The series “Stem Cells in Cancer” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Citrin D, Cotrim AP, Hyodo F, et al. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist 2010;15:360-71. [PubMed]
- Greenberger JS. Radioprotection. In Vivo 2009;23:323-36. [PubMed]
- Hauer-Jensen M, Wang J, Denham JW. Bowel injury: current and evolving management strategies. Semin Radiat Oncol 2003;13:357-71. [PubMed]
- Yeoh E, Horowitz M, Russo A, et al. Effect of pelvic irradiation on gastrointestinal function: a prospective longitudinal study. Am J Med 1993;95:397-406. [PubMed]
- Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. Bioessays 2002;24:91-8. [PubMed]
- Bjerknes M, Cheng H. Gastrointestinal stem cells. II. Intestinal stem cells. Am J Physiol Gastrointest Liver Physiol 2005;289:G381-7. [PubMed]
- Barker N, van Es JH, Jaks V, et al. Very long-term self-renewal of small intestine, colon, and hair follicles from cycling Lgr5+ve stem cells. Cold Spring Harb Symp Quant Biol 2008;73:351-6. [PubMed]
- Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 1999;116:7-14. [PubMed]
- Winton DJ, Ponder BA. Stem-cell organization in mouse small intestine. Proc Biol Sci 1990;241:13-8. [PubMed]
- Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell 2012;11:452-60. [PubMed]
- Rothenberg ME, Nusse Y, Kalisky T, et al. Identification of a cKit(+) colonic crypt base secretory cell that supports Lgr5(+) stem cells in mice. Gastroenterology 2012;142:1195-205.e6.
- Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol 1997;78:219-43. [PubMed]
- Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003-7. [PubMed]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 2008;40:915-20. [PubMed]
- Montgomery RK, Carlone DL, Richmond CA, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A 2011;108:179-84. [PubMed]
- Takeda N, Jain R, LeBoeuf MR, et al. Interconversion between intestinal stem cell populations in distinct niches. Science 2011;334:1420-4. [PubMed]
- Powell AE, Wang Y, Li Y, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 2012;149:146-58. [PubMed]
- Wong VW, Stange DE, Page ME, et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol 2012;14:401-8. [PubMed]
- Zhu L, Gibson P, Currle DS, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 2009;457:603-7. [PubMed]
- Snippert HJ, van Es JH, van den Born M, et al. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology 2009;136:2187-94.e1.
- Potten CS, Booth C, Tudor GL, et al. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 2003;71:28-41. [PubMed]
- Dekaney CM, Rodriguez JM, Graul MC, et al. Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology 2005;129:1567-80. [PubMed]
- Yan KS, Chia LA, Li X, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A 2012;109:466-71. [PubMed]
- Itzkovitz S, Lyubimova A, Blat IC, et al. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat Cell Biol 2012;14:106-14. [PubMed]
- Qiu W, Carson-Walter EB, Liu H, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell 2008;2:576-83. [PubMed]
- Hua G, Thin TH, Feldman R, et al. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology 2012;143:1266-76. [PubMed]
- Muñoz J, Stange DE, Schepers AG, et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J 2012;31:3079-91. [PubMed]
- Qiu W, Leibowitz B, Zhang L, et al. Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene 2010;29:1622-32. [PubMed]
- Buczacki SJ, Zecchini HI, Nicholson AM, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 2013;495:65-9. [PubMed]
- Roth S, Franken P, Sacchetti A, et al. Paneth cells in intestinal homeostasis and tissue injury. PLoS One 2012;7:e38965 [PubMed]
- van Es JH, Sato T, van de Wetering M, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 2012;14:1099-104. [PubMed]
- Van Landeghem L, Santoro MA, Krebs AE, et al. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol 2012;302:G1111-32. [PubMed]
- Dekaney CM, Gulati AS, Garrison AP, et al. Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice. Am J Physiol Gastrointest Liver Physiol 2009;297:G461-70. [PubMed]
- Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res 2004;161:123-36. [PubMed]
- Wang F, Scoville D, He XC, et al. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology 2013;145:383-395.e21.
- Gracz AD, Fuller MK, Wang F, et al. Brief Report CD24 and CD44 mark human intestinal epithelial cell populations with characteristics of active and facultative stem cells. Stem Cells 2013;31:2024-30. [PubMed]
- Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009;459:262-5. [PubMed]
- Stelzner M, Helmrath M, Dunn JC, et al. A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol 2012;302:G1359-63. [PubMed]
- Komarova EA, Kondratov RV, Wang K, et al. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene 2004;23:3265-71. [PubMed]
- Terry NH, Travis EL. The influence of bone marrow depletion on intestinal radiation damage. Int J Radiat Oncol Biol Phys 1989;17:569-73. [PubMed]
- Withers HR, Elkind MM. Radiosensitivity and fractionation response of crypt cells of mouse jejunum. Radiat Res 1969;38:598-613. [PubMed]
- Withers HR, Elkind MM. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med 1970;17:261-7. [PubMed]
- Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell 2007;28:739-45. [PubMed]
- Bennett CB, Lewis LK, Karthikeyan G, et al. Genes required for ionizing radiation resistance in yeast. Nat Genet 2001;29:426-34. [PubMed]
- Gudkov AV, Komarova EA. Pathologies associated with the p53 response. Cold Spring Harb Perspect Biol 2010;2:a001180 [PubMed]
- Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun 2005;331:851-8. [PubMed]
- Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer 2002;2:594-604. [PubMed]
- Yu J, Zhang L, Hwang PM, et al. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 2001;7:673-82. [PubMed]
- el-Deiry WS, Harper JW, O’Connor PM, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 1994;54:1169-74. [PubMed]
- Gottlieb E, Haffner R, King A, et al. Transgenic mouse model for studying the transcriptional activity of the p53 protein: age- and tissue-dependent changes in radiation-induced activation during embryogenesis. Embo J 1997;16:1381-90. [PubMed]
- Komarova EA, Chernov MV, Franks R, et al. Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during normal development and determines radiation and drug sensitivity in vivo. Embo J 1997;16:1391-400. [PubMed]
- Iyer NG, Chin SF, Ozdag H, et al. p300 regulates p53-dependent apoptosis after DNA damage in colorectal cancer cells by modulation of PUMA/p21 levels. Proc Natl Acad Sci U S A 2004;101:7386-91. [PubMed]
- Seoane J, Le HV, Massague J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature 2002;419:729-34. [PubMed]
- Wu WS, Heinrichs S, Xu D, et al. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell 2005;123:641-53. [PubMed]
- Fei P, Bernhard EJ, El-Deiry WS. Tissue-specific induction of p53 targets in vivo. Cancer Res 2002;62:7316-27. [PubMed]
- Westphal CH, Rowan S, Schmaltz C, et al. atm and p53 cooperate in apoptosis and suppression of tumorigenesis, but not in resistance to acute radiation toxicity. Nat Genet 1997;16:397-401. [PubMed]
- Botchkarev VA, Komarova EA, Siebenhaar F, et al. p53 is essential for chemotherapy-induced hair loss. Cancer Res 2000;60:5002-6. [PubMed]
- Gibson RJ, Bowen JM, Inglis MR, et al. Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. J Gastroenterol Hepatol 2003;18:1095-100. [PubMed]
- Bowen JM, Gibson RJ, Stringer AM, et al. Role of p53 in irinotecan-induced intestinal cell death and mucosal damage. Anticancer Drugs 2007;18:197-210. [PubMed]
- Merritt AJ, Potten CS, Kemp CJ, et al. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res 1994;54:614-7. [PubMed]
- Merritt AJ, Allen TD, Potten CS, et al. Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene 1997;14:2759-66. [PubMed]
- Leibowitz BJ, Qiu W, Liu H, et al. Uncoupling p53 Functions in Radiation-Induced Intestinal Damage via PUMA and p21. Mol Cancer Res 2011;9:616-25. [PubMed]
- Kirsch DG, Santiago PM, di Tomaso E, et al. p53 Controls Radiation-Induced Gastrointestinal Syndrome in Mice Independent of Apoptosis. Science 2010;327:593-6. [PubMed]
- Sullivan JM, Jeffords LB, Lee CL, et al. p21 protects “Super p53” mice from the radiation-induced gastrointestinal syndrome. Radiat Res 2012;177:307-10. [PubMed]
- Johnson SM, Torrice CD, Bell JF, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J Clin Invest 2010;120:2528-36. [PubMed]
- Yu H, Shen H, Yuan Y, et al. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood 2010;115:3472-80. [PubMed]
- Shao L, Sun Y, Zhang Z, et al. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood 2010;115:4707-14. [PubMed]
- Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene 2008;27:S71-83. [PubMed]
- Jeffers JR, Parganas E, Lee Y, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 2003;4:321-8. [PubMed]
- Villunger A, Michalak EM, Coultas L, et al. p53- and Drug-Induced Apoptotic Responses Mediated by BH3-Only Proteins Puma and Noxa. Science 2003;302:1036-8. [PubMed]
- Erlacher M, Michalak EM, Kelly PN, et al. BH3-only proteins Puma and Bim are rate-limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood 2005;106:4131-8. [PubMed]
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007;26:1324-37. [PubMed]
- Yu J, Zhang L. Apoptosis in human cancer cells. Curr Opin Oncol 2004;16:19-24. [PubMed]
- Leibowitz B, Yu J. Mitochondrial signaling in cell death via the Bcl-2 family. Cancer Biol Ther 2010;9:417-22. [PubMed]
- Shibue T, Takeda K, Oda E, et al. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev 2003;17:2233-8. [PubMed]
- Rotolo JA, Maj JG, Feldman R, et al. Bax and Bak do not exhibit functional redundancy in mediating radiation-induced endothelial apoptosis in the intestinal mucosa. Int J Radiat Oncol Biol Phys 2008;70:804-15. [PubMed]
- Lindsten T, Ross AJ, King A, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell 2000;6:1389-99. [PubMed]
- Pritchard DM, Potten CS, Korsmeyer SJ, et al. Damage-induced apoptosis in intestinal epithelia from bcl-2-null and bax-null mice: investigations of the mechanistic determinants of epithelial apoptosis in vivo. Oncogene 1999;18:7287-93. [PubMed]
- Pritchard DM, Print C, O’Reilly L, et al. Bcl-w is an important determinant of damage-induced apoptosis in epithelia of small and large intestine. Oncogene 2000;19:3955-9. [PubMed]
- Hoyes KP, Cai WB, Potten CS, et al. Effect of bcl-2 deficiency on the radiation response of clonogenic cells in small and large intestine, bone marrow and testis. Int J Radiat Biol 2000;76:1435-42. [PubMed]
- Watson AJ, Pritchard DM. Lessons from genetically engineered animal models. VII. Apoptosis in intestinal epithelium: lessons from transgenic and knockout mice. Am J Physiol Gastrointest Liver Physiol 2000;278:G1-5. [PubMed]
- Ch’ang HJ, Maj JG, Paris F, et al. ATM regulates target switching to escalating doses of radiation in the intestines. Nat Med 2005;11:484-90. [PubMed]
- Clarke AR, Jones N, Pryde F, et al. 53BP1 deficiency in intestinal enterocytes does not alter the immediate response to ionizing radiation, but leads to increased nuclear area consistent with polyploidy. Oncogene 2007;26:6349-55. [PubMed]
- Ishizuka S, Martin K, Booth C, et al. Poly(ADP-ribose) polymerase-1 is a survival factor for radiation-exposed intestinal epithelial stem cells in vivo. Nucleic Acids Res 2003;31:6198-205. [PubMed]
- Rotolo JA, Mesicek J, Maj J, et al. Regulation of ceramide synthase-mediated crypt epithelium apoptosis by DNA damage repair enzymes. Cancer Res 2010;70:957-67. [PubMed]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med 2004;10:789-99. [PubMed]
- Toft NJ, Winton DJ, Kelly J, et al. Msh2 status modulates both apoptosis and mutation frequency in the murine small intestine. Proc Natl Acad Sci U S A 1999;96:3911-5. [PubMed]
- Sansom OJ, Toft NJ, Winton DJ, et al. Msh-2 suppresses in vivo mutation in a gene dose and lesion dependent manner. Oncogene 2001;20:3580-4. [PubMed]
- Sansom OJ, Bishop SM, Court H, et al. Apoptosis and mutation in the murine small intestine: loss of Mlh1- and Pms2-dependent apoptosis leads to increased mutation in vivo. DNA Repair (Amst) 2003;2:1029-39. [PubMed]
- Sansom OJ, Zabkiewicz J, Bishop SM, et al. MBD4 deficiency reduces the apoptotic response to DNA-damaging agents in the murine small intestine. Oncogene 2003;22:7130-6. [PubMed]
- Sansom OJ, Clarke AR. The ability to engage enterocyte apoptosis does not predict long-term crypt survival in p53 and Msh2 deficient mice. Oncogene 2002;21:5934-9. [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [PubMed]
- Wang Y, Meng A, Lang H, et al. Activation of nuclear factor kappaB In vivo selectively protects the murine small intestine against ionizing radiation-induced damage. Cancer Res 2004;64:6240-6. [PubMed]
- Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004;118:285-96. [PubMed]
- Stenson WF. Prostaglandins and epithelial response to injury. Curr Opin Gastroenterol 2007;23:107-10. [PubMed]
- Houchen CW, Stenson WF, Cohn SM. Disruption of cyclooxygenase-1 gene results in an impaired response to radiation injury. Am J Physiol Gastrointest Liver Physiol 2000;279:G858-65. [PubMed]
- Cohn SM, Schloemann S, Tessner T, et al. Crypt stem cell survival in the mouse intestinal epithelium is regulated by prostaglandins synthesized through cyclooxygenase-1. J Clin Invest 1997;99:1367-79. [PubMed]
- Antoch MP, Kondratov RV. Pharmacological modulators of the circadian clock as potential therapeutic drugs: focus on genotoxic/anticancer therapy. Handb Exp Pharmacol 2013;217:289-309. [PubMed]
- Karpowicz P, Zhang Y, Hogenesch JB, et al. The circadian clock gates the intestinal stem cell regenerative state. Cell Rep 2013;3:996-1004. [PubMed]
- Plikus MV, Vollmers C, de la Cruz D, et al. Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proc Natl Acad Sci U S A 2013;110:E2106-15. [PubMed]
- Gorbacheva VY, Kondratov RV, Zhang R, et al. Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proc Natl Acad Sci U S A 2005;102:3407-12. [PubMed]
- Hu Y, Spengler ML, Kuropatwinski KK, et al. Selenium is a modulator of circadian clock that protects mice from the toxicity of a chemotherapeutic drug via upregulation of the core clock protein, BMAL1. Oncotarget 2011;2:1279-90. [PubMed]
- Mills JC, Gordon JI. The intestinal stem cell niche: there grows the neighborhood. Proc Natl Acad Sci U S A 2001;98:12334-6. [PubMed]
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol 2005;21:605-31. [PubMed]
- Quante M, Wang TC. Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology (Bethesda) 2008;23:350-9. [PubMed]
- Francois A, Milliat F, Guipaud O, et al. Inflammation and immunity in radiation damage to the gut mucosa. Biomed Res Int 2013;2013:123241.
- Booth D, Potten CS. Protection against mucosal injury by growth factors and cytokines. J Natl Cancer Inst Monogr 2001;29:16-20. [PubMed]
- Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001;293:293-7. [PubMed]
- Rotolo J, Stancevic B, Zhang J, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J Clin Invest 2012;122:1786-90. [PubMed]
- Wang J, Boerma M, Fu Q, et al. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol 2007;13:3047-55. [PubMed]
- Gulati AS, Ochsner SA, Henning SJ. Molecular properties of side population-sorted cells from mouse small intestine. Am J Physiol Gastrointest Liver Physiol 2008;294:G286-94. [PubMed]
- Santana P, Pena LA, Haimovitz-Friedman A, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell 1996;86:189-99. [PubMed]
- Qiu W, Wang X, Buchanan M, et al. ADAR1 is essential for intestinal homeostasis and stem cell maintenance. Cell death & disease 2013;4:e599 [PubMed]
- Qiu W, Wu B, Wang X, et al. PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J Clin Invest 2011;121:1722-32. [PubMed]
- Dirisina R, Katzman RB, Goretsky T, et al. p53 and PUMA independently regulate apoptosis of intestinal epithelial cells in patients and mice with colitis. Gastroenterology 2011;141:1036-45. [PubMed]
- Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009;457:608-11. [PubMed]
- Qiu W, Carson-Walter EB, Kuan SF, et al. PUMA suppresses intestinal tumorigenesis in mice. Cancer Res 2009;69:4999-5006. [PubMed]
- Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 2003;189:1-20. [PubMed]
- Lam W, Bussom S, Guan F, et al. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci Transl Med 2010;2:45ra59 [PubMed]
- Hagiwara A, Nakayama F, Motomura K, et al. Comparison of expression profiles of several fibroblast growth factor receptors in the mouse jejunum: suggestive evidence for a differential radioprotective effect among major FGF family members and the potency of FGF1. Radiat Res 2009;172:58-65. [PubMed]
- Houchen CW, George RJ, Sturmoski MA, et al. FGF-2 enhances intestinal stem cell survival and its expression is induced after radiation injury. Am J Physiol 1999;276:G249-58. [PubMed]
- Nakayama F, Hagiwara A, Umeda S, et al. Post treatment with an FGF chimeric growth factor enhances epithelial cell proliferation to improve recovery from radiation-induced intestinal damage. Int J Radiat Oncol Biol Phys 2010;78:860-7. [PubMed]
- Wilkins HR, Ohneda K, Keku TO, et al. Reduction of spontaneous and irradiation-induced apoptosis in small intestine of IGF-I transgenic mice. Am J Physiol Gastrointest Liver Physiol 2002;283:G457-64. [PubMed]
- Zheng H, Wang J, Koteliansky VE, et al. Recombinant soluble transforming growth factor beta type II receptor ameliorates radiation enteropathy in mice. Gastroenterology 2000;119:1286-96. [PubMed]
- Booth C, Booth D, Williamson S, et al. Teduglutide ([Gly2]GLP-2) protects small intestinal stem cells from radiation damage. Cell Prolif 2004;37:385-400. [PubMed]
- Kim KA, Kakitani M, Zhao J, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 2005;309:1256-9. [PubMed]
- Zsebo KM, Smith KA, Hartley CA, et al. Radioprotection of mice by recombinant rat stem cell factor. Proc Natl Acad Sci U S A 1992;89:9464-8. [PubMed]
- Leigh BR, Khan W, Hancock SL, et al. Stem cell factor enhances the survival of murine intestinal stem cells after photon irradiation. Radiat Res 1995;142:12-5. [PubMed]
- Huang FS, Kemp CJ, Williams JL, et al. Role of epidermal growth factor and its receptor in chemotherapy-induced intestinal injury. Am J Physiol Gastrointest Liver Physiol 2002;282:G432-42. [PubMed]
- Farrell CL, Bready JV, Rex KL, et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res 1998;58:933-9. [PubMed]
- Fukata M, Abreu MT. Pathogen recognition receptors, cancer and inflammation in the gut. Curr Opin Pharmacol 2009;9:680-7. [PubMed]
- Marques R, Boneca IG. Expression and functional importance of innate immune receptors by intestinal epithelial cells. Cell Mol Life Sci 2011;68:3661-73. [PubMed]
- Gudkov AV, Komarova EA. Radioprotection: smart games with death. J Clin Invest 2010;120:2270-3. [PubMed]
- Neish AS. TLRS in the gut. II. Flagellin-induced inflammation and antiapoptosis. Am J Physiol Gastrointest Liver Physiol 2007;292:G462-6. [PubMed]
- Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 2008;320:226-30. [PubMed]
- Saha S, Bhanja P, Liu L, et al. TLR9 agonist protects mice from radiation-induced gastrointestinal syndrome. PLoS One 2012;7:e29357 [PubMed]
- Mustata G, Li M, Zevola N, et al. Development of small-molecule PUMA inhibitors for mitigating radiation-induced cell death. Curr Top Med Chem 2011;11:281-90. [PubMed]
- Charvet C, Wissler M, Brauns-Schubert P, et al. Phosphorylation of Tip60 by GSK-3 determines the induction of PUMA and apoptosis by p53. Mol Cell 2011;42:584-96. [PubMed]
- Nayak G, Cooper GM. p53 is a major component of the transcriptional and apoptotic program regulated by PI 3-kinase/Akt/GSK3 signaling. Cell death & disease 2012;3:e400 [PubMed]
- Sémont A, François S, Mouiseddine M, et al. Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv Exp Med Biol 2006;585:19-30. [PubMed]
- Sémont A, Mouiseddine M, François A, et al. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ 2010;17:952-61. [PubMed]
- Saha S, Bhanja P, Kabarriti R, et al. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One 2011;6:e24072 [PubMed]
- Chang YH, Lin LM, Lou CW, et al. Bone marrow transplantation rescues intestinal mucosa after whole body radiation via paracrine mechanisms. Radiother Oncol 2012;105:371-7. [PubMed]
- Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701-5. [PubMed]
- Goodell MA, Jackson KA, Majka SM, et al. Stem cell plasticity in muscle and bone marrow. Ann N Y Acad Sci 2001;938:208-18; discussion 18-20. [PubMed]
- Brazelton TR, Rossi FM, Keshet GI, et al. From marrow to brain: expression of neuronal phenotypes in adult mice. Science 2000;290:1775-9. [PubMed]
- Okamoto R, Yajima T, Yamazaki M, et al. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med 2002;8:1011-7. [PubMed]
- Okamoto R, Matsumoto T, Watanabe M. Regeneration of the intestinal epithelia: regulation of bone marrow-derived epithelial cell differentiation towards secretory lineage cells. Hum Cell 2006;19:71-5. [PubMed]
- Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369-77. [PubMed]


