
Chromosomal and telomeric biomarkers of normal tissue injury to evaluate risk of degenerative health effects (secondary malignancy, cardiovascular disease) post radiation therapy
Chromosomal inversions
Structural chromosome aberrations are associated with virtually all known cancers, which to date have consisted primarily of inter-chromosomal rearrangements (i.e., translocations between chromosomes) identified by multicolor fluorescence in situ hybridization (FISH) techniques (1). Of relevance in this regard, is a large whole genome sequencing effort that recently reported radiation therapy-associated second malignancies (of different tumor types) display distinctive mutational signatures, which included small deletions and an increased frequency of putative inversions (2). Interestingly, interstitial deletions and inversions represent alternative outcomes of the same recombinational process; i.e., resolution of two intra-chromosomal double-strand breaks (DSBs)—either with loss (deletion), or after reversal and reinsertion (inversion) of the intervening fragment—thus, they would be expected to occur at approximately similar frequencies. However, because inversions represent rearrangements within chromosomes, they are notoriously difficult to detect, having been done primarily with low resolution banding approaches (e.g., G, C, R, Q, M, and DAPI), and so inversions are largely underrepresented and little appreciated.
Directional Genomic Hybridization (dGH) is a patented cytogenomics based technology (KromaTiD, Inc.) for cell-by-cell detection and/or discovery of all structural variants (SVs), including inversions. The dGH methodology extends the strand-specific strategy of chromosome orientation FISH (CO-FISH) (3) to encompass bioinformatics-driven design of unique directional probe sets that hybridize along one, and only one, chromatid of targeted and prepared metaphase chromosomes, such that inverted fragments (two breakpoints) are easily visualized as segments of signal “switching” to the sister (opposite) chromatid (4). Therefore, dGH circumvents many of the difficulties associated with inversion detection and further, it facilitates high-resolution detection of SVs, most notably those that would otherwise be missed by sequencing or genomic arrays (particularly, small inversions); i.e., dGH greatly improves accurate detection (and validation) of otherwise cryptic rearrangements within the genome (manuscript in preparation). Utilizing dGH, we have demonstrated the dose-dependent induction of inversions following exposure to radiations of vastly different ionization densities [i.e., linear energy transfer (LET)] (5). We have also found inversions to be transmissible—even 60 years post exposure—and that compared to translocations, inversions increase at a greater rate per unit dose (5) (and manuscripts in preparation).
Whereas inversions are induced by exposure to ionizing radiations (IRs) and are mediated by non-homologous end-joining, sister chromatid exchange (SCE)—an event that results in a single signal “switch” (one breakpoint) with dGH—is associated with DNA replication, where resolution via recombination and crossover results in template switching between sister chromatids (6). Although inversion and SCE represent two very distinct processes and products, they are often difficult to definitively distinguish cytologically, especially when such rearrangements occur near chromosomal termini. In addition to generally restricted resolution in this region, elevated rates of mitotic recombination near terminal chromosomal regions (7), as well as within telomeres themselves (T-SCE) (8) have been reported by us and others. Telomeres, the physical ends of linear chromosomes, are composed of tandem arrays of repetitive DNA sequence that serve to protect natural chromosomal ends and prevent their detection as broken DNA, thereby evading inappropriate damage responses and preserving genome stability (9). Utilizing a particularly useful property of telomeric sequences, that of their conserved orientation [5'-(TTAGGG)n-3'], it is possible to determine the directionality of other DNA sequences of interest (10). In an effort to clarify and interpret unusually high frequencies of single terminal dGH signal “switches” (“apparent” SCE), we recently extended this strategy, i.e., using telomeres as points of reference (cis vs. trans configuration), by combining it with whole chromosome dGH chromatid paints—an approach termed Telomere-dGH (Telo-dGH)—to not only routinely identify SCEs, but also to reliably distinguish these recombination events from terminal exchange events, specifically inversions (Figure 1) (11). In addition and importantly, Telo-dGH enables evaluation of telomere length dynamics in the same samples.
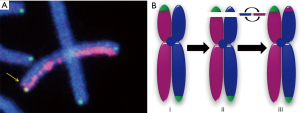
Our on-going research program includes using Telo-dGH for assessing the health effects of spaceflight in twin and unrelated astronauts (NASA). Similarly, in a cohort of US veterans exposed to fallout during nuclear bomb testing post WWII, we have recently demonstrated the utility of Telo-dGH for retrospective biodosimetry (National Institutes of Health/National Cancer Institute); manuscripts in preparation.
Telomeres
While it is well established that telomere length diminishes with cellular division and normal aging due to the end-replication problem, it is also becoming increasingly appreciated that inflammation and oxidative stress, as well as a variety of lifestyle factors (including nutritional, psychological, physical stresses) and environmental exposures (e.g., IRs) also contribute to telomere erosion. Although we and others have shown that telomere length and telomerase activity (the enzyme capable of counteracting telomere attrition in continuously dividing cells such as germ, stem and cancer cells) are influenced by IRs, (12,13), the effects of low dose exposure on human telomere length dynamics (i.e., with time) are much less well understood and appreciated [reviewed in (14)]. Telomeres have been proposed as “hallmarks of radiosensitivity” (15), as short telomeres enhance radiosensitivity (16,17). Of particular interest are reports of significantly shortened telomeres in the peripheral blood of radiotherapy patients following treatment for a variety of cancer types—within a relatively short span of time (3 months or less), and specifically within the shortest telomere populations (18). Long-term radiation effects including dose-dependent telomere shortening have also been reported in exposed populations following the Chernobyl nuclear accident (19). Interestingly, low LET X-ray and low energy (high LET) protons induced different telomeric responses in human fibroblasts, in that telomeres were shortened 24 h post X-ray exposure and associated with anaphase bridges and dicentrics, while high LET protons evoked telomere lengthening at 24 and 96 h (20).
Taken together, accumulating evidence convincingly supports telomere length maintenance as an informative general measure of health (21), in that it represents a key integrating component for the cumulative effects of genetic, environmental, and lifestyle factors on aging and age related diseases—one that captures the radiological response as well. Thus, the rate at which telomeres shorten provides an informative biomarker of biological aging and further, erosion of telomere length can be linked to age-related degenerative pathologies ranging from reduced immune function, loss of fertility, idiopathic pulmonary fibrosis and dementia, to cardiovascular disease (CVD) (22) and cancer (23).
Atherosclerosis, the major cause of CVD, is an age-related disease characterized by systemic oxidative stress and low grade chronic inflammation. Inflammation and the associated proliferation of cells during infection and disease result in loss of telomere sequence via the accompanying oxidative stress (24). Evidence that oxidative stress drives telomere shortening in humans led to the suggestion that telomere length in peripheral blood cells might be a valuable biomarker for age related risk of mortality and morbidity and, specifically, for CVDs (25). Indeed, the first meta-analysis integrating data from 24 individual studies on the link between telomere length and CVD reported, as expected, an inverse relationship between leukocyte telomere length and risk of coronary heart disease (26); this association remained significant when confined to prospective studies and was independent of conventional vascular risk factors.
Importantly, IR exposure induces mitochondrial dysfunction and excess oxidative stress that persists long after the initial exposure (27). IR-induced chronic oxidative stress has also been implicated in delayed/on-going chromosomal instability (28). The G-rich nature of TTAGGG repeats renders telomeres highly susceptible to oxidative damage, and that oxidative stress accelerates telomere shortening is well accepted (29). Further, oxidative guanine base damage has recently been shown to regulate telomerase activity, in addition to disrupting the critical telomere binding factors TRF1 and TRF2 (30). The presence of such DNA damage within telomeres interferes with their efficient replication (31). Moreover, oxidative damage to telomeric DNA tends to accumulate because it is repaired less efficiently than other regions of the genome (32). Telomeres and adjacent sub-telomeric regions (minimally ~100 kb) have also been shown to be sensitive to I-SceI-induced DSBs due to deficiency in their repair (33,34). In stimulated human peripheral blood mononuclear cells (PBMCs) exposed to a total dose of 1 Gy (gamma-rays) delivered at low dose rate (4.9 cGy/h), we observed significantly elevated telomerase activity 2 and 5 days post exposure, which was associated with enrichment of putative stem cell compartments via both mobilization (akin to wound healing) and IR-induced reprogramming (13,35). Even so, telomere length was significantly shortened 5 days post low dose rate exposure, as well as at 2 and 5 days post acute exposure (1 Gy), highlighting novel molecular functions of telomerase, as well as important implications for promoting instability, repopulation and second malignancy.
Clinical/translational relevance and application
Intensity-modulated radiation therapy (IMRT) uses enhanced planning treatment software to more precisely target difficult-to-reach tumors (e.g., prostate), allowing delivery of much higher doses to the tumor, while minimizing exposures to surrounding normal tissue. However, while spared high doses, there is growing concern that a much larger volume of normal tissue is unavoidably exposed to low doses of IR. Further, because IMRT is particularly useful for treating tumors close to critical structures, it is used to treat cancers of the head and neck, and central nervous system. For example, IMRT is used to treat most types of pediatric malignancy—including brain, the most common solid tumor of childhood. Increased risk of secondary malignancy and other age-related degenerative pathologies (e.g., CVD) post radiation therapy are certainly of concern, particularly for pediatric patients due to their longer post treatment lifespan.
We propose advancing Telo-dGH as a prospective “personalized” approach of monitoring normal tissue injury, and therefore future risk, associated with radiation therapy—regardless of tumor type or treatment modality (e.g., IMRT, protons or carbon ions). Relevant clinical applications currently being conducted and/or initiated in our laboratory include IMRT for prostate cancer (Dr. Gregory P. Swanson M.D., Baylor Scott and White Clinic; KromaTiD, Inc.), and IMRT vs. proton therapy for childhood brain tumors (National Institutes of Health/National Cancer Institute). The approach is straightforward and non-invasive. Individual patient’s blood is collected before, immediately after, and ideally at several times post-treatment, but minimally at approximately 3 months. Samples are processed for Telo-dGH and analyzed for chromosomal (inversions, translocations) and telomeric (length dynamics) biomarkers of normal tissue injury. Utilizing Telo-dGH for routine monitoring of radiation therapy patients to assess radiosensitivity (toxicity), as well as risk of second malignancy and other degenerative health effects (e.g., CVD) following treatment, will be extremely valuable for improving understanding—and personalized management—of increased risk across a variety of adult and pediatric cancers.
Acknowledgments
The authors wish to thank Dr. Michael Cornforth for helpful discussions, and KromaTiD, Inc. (Fort Collins, CO, USA) for Telo-dGH probe development.
Funding: Support from NASA (NNX14AB02G; NNX14AH51G) is gratefully acknowledged.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Durante, Giusi I. Forte, Giorgio Russo) for the series “Radiobiological models towards a personalized radiation oncology” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.05.20). The series “Radiobiological models towards a personalized radiation oncology” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mitelman F. Recurrent chromosome aberrations in cancer. Mutat Res 2000;462:247-53. [Crossref] [PubMed]
- Behjati S, Gundem G, Wedge DC, et al. Mutational signatures of ionizing radiation in second malignancies. Nat Commun 2016;7:12605. [Crossref] [PubMed]
- Bailey SM, Goodwin EH, Cornforth MN. Strand-specific fluorescence in situ hybridization: the CO-FISH family. Cytogenet Genome Res 2004;107:14-7. [Crossref] [PubMed]
- Ray FA, Zimmerman E, Robinson B, et al. Directional genomic hybridization for chromosomal inversion discovery and detection. Chromosome Res 2013;21:165-74. [Crossref] [PubMed]
- Ray FA, Robinson E, McKenna M, et al. Directional genomic hybridization: inversions as a potential biodosimeter for retrospective radiation exposure. Radiat Environ Biophys 2014;53:255-63. [Crossref] [PubMed]
- Painter RB. A replication model for sister-chromatid exchange. Mutat Res 1980;70:337-41. [Crossref] [PubMed]
- Cornforth MN, Eberle RL. Termini of human chromosomes display elevated rates of mitotic recombination. Mutagenesis 2001;16:85-9. [Crossref] [PubMed]
- Bailey SM, Brenneman MA, Goodwin EH. Frequent recombination in telomeric DNA may extend the proliferative life of telomerase-negative cells. Nucleic Acids Res 2004;32:3743-51. [Crossref] [PubMed]
- de Lange T. How telomeres solve the end-protection problem. Science 2009;326:948-52. [Crossref] [PubMed]
- Bailey SM, Meyne J, Cornforth MN, et al. A new method for detecting pericentric inversions using COD-FISH. Cytogenetics and Cell Genetics 1996;75:248-53. [Crossref] [PubMed]
- McKenna MJ, Robinson E, Goodwin EH, et al. Telomeres and NextGen CO-FISH: Directional Genomic Hybridization (Telo-dGH™). Methods Mol Biol 2017;1587:103-12. [Crossref] [PubMed]
- Neuhof D, Ruess A, Wenz F, et al. Induction of telomerase activity by irradiation in human lymphoblasts. Radiation research 2001;155:693-7. [Crossref] [PubMed]
- Sishc BJ, Nelson CB, McKenna MJ, et al. Telomeres and Telomerase in the Radiation Response: Implications for Instability, Reprograming, and Carcinogenesis. Front Oncol 2015;5:257. [Crossref] [PubMed]
- Sridharan DM, Asaithamby A, Bailey SM, et al. Understanding cancer development processes after HZE-particle exposure: roles of ROS, DNA damage repair and inflammation. Radiation research 2015;183:1-26. [Crossref] [PubMed]
- Ayouaz A, Raynaud C, Heride C, et al. Telomeres: hallmarks of radiosensitivity. Biochimie 2008;90:60-72. [Crossref] [PubMed]
- Wong KK, Chang S, Weiler SR, et al. Telomere dysfunction impairs DNA repair and enhances sensitivity to ionizing radiation. Nature Genetics 2000;85-8. [PubMed]
- Goytisolo FA, Samper E, Martin-Caballero J, et al. Short telomeres result in organismal hypersensitivity to ionizing radiation in mammals. J Exp Med 2000;192:1625-36. [Crossref] [PubMed]
- Maeda T, Nakamura K, Atsumi K, et al. Radiation-associated changes in the length of telomeres in peripheral leukocytes from inpatients with cancer. Int J Radiat Biol 2013;89:106-9. [Crossref] [PubMed]
- Ilyenko I, Lyaskivska O, Bazyka D. Analysis of relative telomere length and apoptosis in humans exposed to ionising radiation. Experimental oncology 2011;33:235-8. [PubMed]
- Berardinelli F, Antoccia A, Buonsante R, et al. The role of telomere length modulation in delayed chromosome instability induced by ionizing radiation in human primary fibroblasts. Environmental and molecular mutagenesis 2013;54:172-9. [Crossref] [PubMed]
- Borresen EC, Brown DG, Harbison G, et al. A Randomized Controlled Trial to Increase Navy Bean or Rice Bran Consumption in Colorectal Cancer Survivors. Nutr Cancer 2016;68:1269-80. [Crossref] [PubMed]
- Mourkioti F, Kustan J, Kraft P, et al. Role of telomere dysfunction in cardiac failure in Duchenne muscular dystrophy. Nature cell biology 2013;15:895-904. [Crossref] [PubMed]
- Shay JW. Are short telomeres hallmarks of cancer recurrence? Clin Cancer Res 2014;20:779-81. [Crossref] [PubMed]
- VonZglinicki T, Pilger R, Sitte N. Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radical Biology and Medicine 2000;28:64-74. [Crossref] [PubMed]
- von Zglinicki T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci 2000;908:99-110. [Crossref] [PubMed]
- Haycock PC, Heydon EE, Kaptoge S, et al. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 2014;349:g4227. [Crossref] [PubMed]
- Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer letters 2012;327:48-60. [Crossref] [PubMed]
- Limoli CL, Giedzinski E. Induction of chromosomal instability by chronic oxidative stress. Neoplasia 2003;5:339-46. [Crossref] [PubMed]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci 2002;27:339-44. [Crossref] [PubMed]
- Fouquerel E, Lormand J, Bose A, et al. Oxidative guanine base damage regulates human telomerase activity. Nat Struct Mol Biol 2016;23:1092-100. [Crossref] [PubMed]
- Tchirkov A, Lansdorp PM. Role of oxidative stress in telomere shortening in cultured fibroblasts from normal individuals and patients with ataxia-telangiectasia. Human molecular genetics 2003;12:227-32. [Crossref] [PubMed]
- Rochette PJ, Brash DE. Human telomeres are hypersensitive to UV-induced DNA Damage and refractory to repair. PLoS genetics 2010;6:e1000926 [Crossref] [PubMed]
- Kulkarni A, Zschenker O, Reynolds G, et al. Effect of telomere proximity on telomere position effect, chromosome healing, and sensitivity to DNA double-strand breaks in a human tumor cell line. Molecular and cellular biology 2010;30:578-89. [Crossref] [PubMed]
- Miller D, Reynolds GE, Mejia R, et al. Subtelomeric regions in mammalian cells are deficient in DNA double-strand break repair. DNA repair 2011;10:536-44. [Crossref] [PubMed]
- Gao X, Sishc BJ, Nelson CB, et al. Radiation-Induced Reprogramming of Pre-Senescent Mammary Epithelial Cells Enriches Putative CD44(+)/CD24(-/low) Stem Cell Phenotype. Front Oncol 2016;6:138. [Crossref] [PubMed]


