
What’s new in the treatment of advanced small-cell lung cancer
Introduction
Although the incidence of lung cancer has been declining since 2000, it has remained the leading cause of cancer-related mortality worldwide for the last 10 years for both sexes (1,2). Small-cell lung cancer (SCLC), which accounts for approximately 15% of lung cancers, continues to have a dismal prognosis due to its aggressive biological characteristics, with an overall 5-year survival rate of less than 10% (3). SCLC is highly sensitive to initial chemotherapy; however, nearly all patients eventually relapse. The only FDA-approved treatment for relapsed SCLC in the past 2 decades has been single-agent topotecan, but it has a poor response rate (7%), and its survival rate is unacceptable (4,5).
Approximately 30% of patients are classified as limited-stage (LS) at the time of diagnosis. The current standard of care for LS-SCLC is cisplatin or carboplatin plus etoposide with concurrent thoracic radiation performed early during the first two cycles of chemotherapy. Recently, more emerging data have validated the role of surgical resection in LS-SCLC (6-9), especially for the T1-2N0M0 cases, and it is accepted by the National Comprehensive Cancer Network (NCCN) and ASCO guidelines (10). Compared to LS-SCLC, except for first-line chemotherapy, follow-up treatments for extensive-stage SCLC (ES-SCLC) have made slow progress in recent years, but there still some bright spots. Prophylactic cranial irradiation (PCI) should be considered for patients with LS- or ES-SCLC with favorable performance scores, as it has been shown to decrease the risk of intracranial recurrence and improve overall survival (OS). However, due to the risk of neurocognitive decline, the role of PCI in resected LS-SCLC is controversial (11).
Recently, with the increased attention on drug discovery and the development of precision medicine, several novel therapies for SCLC have emerged. Here, we emphasize novel promising therapies for ES-SCLC (Table 1).
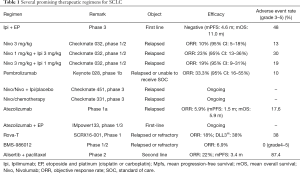
Full table
Checkpoint blockade immunotherapy
In theory, checkpoint blockade immunotherapy could be the most promising treatment for ES-SCLC and alter its standard of care for the following reasons: (I) whole-genome and transcriptome sequencing results highlighted a high frequency of somatic mutation rates in SCLC (12,13), which could theoretically induce a higher neoantigen burden. Neoantigen refers to a class of potential cancer rejection antigens which could be recognized by effective immune cells and prime the immune system (14). Considering this biological feature of SCLC, patients’ immune systems could attack tumor cells persistently during therapy with checkpoint inhibitors, such as ipilimumab (anti-CTLA4), nivolumab (anti-PD-1), pembrolizumab (anti-PD-1) and atezolizumab (anti-PD-L1); (II) it is generally considered that chemotherapy could enhance the diversity of neoantigen expression (15). Coupled with SCLC’s high sensitivity to chemotherapy, the combination of cytotoxic drugs and checkpoint-targeted immunotherapy could result in ideal treatment effects.
In this context, ipilimumab (anti-CTLA4) set the trend for immunotherapy for SCLC. In 2013, Reck and colleagues reported findings from a phase 2 trial that investigated the efficacy of ipilimumab in combination with paclitaxel and carboplatin as a first-line therapy for ES-SCLC. However, there were no significant differences between the control and ipilimumab groups (16). The first phase 3 trial regarding immunotherapy for SCLC was published (17); in it, 1,132 patients with ES-SCLC were randomly assigned to receive either etoposide or platinum (cisplatin or carboplatin) for four cycles alone or together with ipilimumab. Disappointingly, the trial failed at the interim analysis. The OS of the combined-treatment group was not improved (HR, 0.94; 95% CI, 0.81–1.09). The main reasons for failure were attributed to: (I) rapid tumor growth and inferior performance status score of patients with ES-SCLC, which led to poor tolerance to immune-checkpoint inhibitors; (II) excessive patient dropout, which affected clinical outcomes; and (III) the unsuitability of ipilimumab for use after chemotherapy, which regulates the amplitude of the early stages of T cell activation relative to PD-1 pathway blockade agents (18).
Fortunately, the PD-1 antibody (nivolumab or pembrolizumab) showed antitumor activity with durable responses and manageable safety profiles in early-stage clinical trials. A phase 1/2 trial (checkmate 032), in which nivolumab (nivo) was used alone or in combination with ipilimumab (ipi) to treat relapsed SCLC, showed that an objective response was achieved in 10 (10%) of 98 patients receiving nivo 3 mg/kg, 1 (33%) of 3 patients receiving nivo 1 mg/kg plus ipi 1 mg/kg, 14 (23%) of 61 patients receiving nivo 1 mg/kg plus ipi 3 mg/kg, and 10 (19%) of 54 patients receiving nivo 3 mg/kg plus ipi 1 mg/kg (19). These data demonstrated a promising new treatment approach for ES-SCLC. The subsequent design of a phase 3 randomized controlled trial (Checkmate 451) was reported at the 2016 ASCO annual meeting, which evaluated nivo monotherapy, nivo plus ipi followed by nivo monotherapy, and placebo as maintenance therapy for ED-SCLC after first-line chemotherapy (20). Another ongoing phase 3 trial (Checkmate 331) is expected to investigate further the efficacy of nivo for ES-SCLC. Keynote 028 is an ongoing non-randomized Phase 1b basket trial, and the updated survival results have been published. In keynote 028, 24 previously treated ES-SCLC patients, who were PD-L1 expression positive, received pembrolizumab and had an objective response rate (ORR) of 33.3%, a median progression-free survival (mPFS) of 1.9 months, and a median OS (mOS) of 9.7 months (21).
For PD-L1 antibody, atezolizumab, also show its safety and clinical efficacy in a phase 1a study (22); 17 patients enrolled, ORR was 5.9% and immune-related response evaluation criteria in solid tumors (irRECIST) based ORR was 17.6%. Another ongoing phase I/III trail (IMpower133) try to investigate the efficacy and safety of atezolizumab plus chemotherapy in treatment-naive patients with ES-SCLC (23).
As shown above, recent advances in the development of immunotherapy against non-small-cell lung cancer (NSCLC) contrast sharply with the minor progress in ES-SCLC and the treatment efficacy is far from satisfaction. The strategy of the application of checkpoint inhibitors for SCLC is still a major unresolved issue: (I) in the wake of phase of immunotherapy 2.0, how to select potential benefit population becomes the prime target; (II) which is a better choice, monotherapy or combined? To answer the former question, we need more fundamental researches which could be summed up into two aspects. One is the tumor microenvironment, as Chen proposed the cancer-immune phenotypes and the insights to T cell exhaustion (24-26). And the molecular mechanism correlation, as TP53 and KRAS mutation predicts response to PD-1 blockade in NSCLC (27).
ADCs
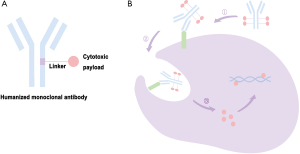
Antibody-drug conjugates (ADCs) comprise cytotoxic drugs conjugated to a humanized monoclonal antibody directed at antigens overexpressed on a tumor cell’s surface (28). The conjugated antibodies are supposed to deliver a cytotoxic effect selectively to a tumor; their purpose is to improve the clinical benefit and minimize the systemic toxicity of traditional chemotherapy (Figure 1). To date, two clinical trials to our knowledge have published results regarding ADCs targeting ES-SCLS.
Rovalpituzumab tesirine (Rova-T)
Through next-generation sequencing, inactivating mutations in TP53 and RB1 have been observed in nearly all SCLC specimens (13). Mutations in RB1 are highly associated with elevated expression of achaete-scute complex homolog 1 (ASCL1). ASCL1 is a basic-helix-loop-helix transcription factor that plays a pivotal role in neuroendocrine differentiation and correlates with tumor-initiating capacity (29,30). By combining whole-genome microarray expression analysis and ChIP-Seq data, delta-like 3 (DLL3) is transcriptionally regulated downstream of the ASCL1. DLL3 is one of the mammalian Notch pathway family ligands, which predominantly localizes to the Golgi apparatus (31). Notch pathway activation acts as anti-oncogenic stimulus in SCLC (32). DLL3 interacts with Notch1 and DLL1 in the Golgi apparatus, retaining and/or redirecting them to endosomes for destruction and thereby preventing them from reaching the cell surface where they can activate Notch signaling in trans (33). DLL3 seems to be an inhibitor of the Notch signal pathway, and the TP53-RB1-ASCL1-Notch signaling axis appears to drive neuroendocrine differentiation (34). Hence, DLL3 could be a potential target for ADCs due to its overexpression in neuroendocrine tumor cells (35).
Saunders and his colleagues (36) established an ADC, Rova-T, to target DLL3+ tumor cells. Rova-T consists of a humanized anti-DLL3 monoclonal antibody and a DNA-damaging pyrrolobenzodiazepine (PBD) dimer toxin. In the SCLC patient-derived xenograft (PDX) model, Rova-T induced durable tumor regression over 4 months and effectively inhibited tumor initial cells (TICs) compared to the activity of chemotherapy regimens. Additionally, a subsequent phase 1 clinical trial was recently completed, in which Rova-T was used to treat recurrent or refractory SCLC. Rova-T led to an ORR in 11 (18%) of 60 evaluable patients, mPFS of 2.8 months, mOS of 4.6 months, and a 1-year survival rate of 15%. In addition, in patients with DLL3 expression >50%, the ORR was 38%, mPFS was 4.3 months, mOS was 5.8 months, and 1-year survival rate was 29% (37). This promising result provided strong support for further investigation of Rova-T. The DLL3 expression level, as a biomarker, should direct the development of subsequent clinical trials.
BMS-986012
At the 2016 EMSO meeting, results of another phase 1/2 trial regarding ADCs for ES-SCLC were published. BMS-986012 is a fully humanized monoclonal antibody with enhanced ADCs that specifically target Fucosyl-GM1 (Fuc-GM1). Fuc-GM1 is a sphingolipid monosialoganglioside and tumor-associated antigen with a high prevalence in SCLC, while its expression is minimal in most normal tissues. Patients with relapsed/refractory SCLC after at least one line of prior therapy were enrolled and 29 patients were treated across all doses. One complete remission (CR), one partial remission (PR), and four stable diseases (SDs) were observed during the procedure, and no dose-limiting toxicities or treatment-related grade 4/5 adverse events occurred (38).
Alisertib
Alisertib is a selective inhibitor of aurora A kinase (AAK). AAK is essential for mitosis (39,40). A previous study showed a high expression of AAK in SCLC and the depressive effect of cell proliferation through knocking down the aurora A gene (40). In a five-arm phase 1/2 study, 48 patients with SCLC with either refractory or recurrent disease received at least one dose of alisertib. The ORR was 21%, and the mPFS was 2.1 months (41). This result showed promising antitumor activity with a manageable safety profile for ES-SCLC. A subsequent randomized phase 2 study has been completed (42). In it, 178 patients were randomly assigned to receive either alisertib and paclitaxel or placebo and paclitaxel. The median PFS was 3.32 months for the alisertib group versus 2.17 months for the placebo group [hazard ratio (HR), 0.77; 95% CI, 0.557–1.067; P=0.113]. In a subgroup of patients with either refractory or relapsed disease, mPFS was 2.86 months versus 1.64 months (HR, 0.659; 95% CI, 0.442–0.983; P=0.0372). Interestingly, c-MYC protein expression showed a strong association with improved PFS, which needs further investigation.
At present, other small-molecule inhibitors, such as sunitinib (a multikinase inhibitor) (43), veliparib (a poly-ADP-ribose polymerase inhibitor) (44), and roniciclib (a cyclin-dependent kinase inhibitor) (45), have not shown any significant improvement for ES-SCLC.
Conclusions
With the increasing focus on systemic therapy for SCLC, several promising regimens have been developed. Despite the unsatisfactory situation, immunotherapy is the most promising; however, problems related to its exorbitant costs and the selection of suitable patients remain to be resolved. Additionally, ADCs and TKIs may result in unexpected and surprising outcomes.
Acknowledgments
Funding: This work was supported by grants from the National Nature Science Foundation of China (81673031).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.05.38). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Pietanza MC. Using a Population-Based Analysis to Determine the Management and Treatment of Early-Stage Small-Cell Lung Cancer. J Clin Oncol 2016;34:1027-9. [Crossref] [PubMed]
- O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441-7. [Crossref] [PubMed]
- Horita N, Yamamoto M, Sato T, et al. Topotecan for Relapsed Small-cell Lung Cancer: Systematic Review and Meta-Analysis of 1347 Patients. Sci Rep 2015;5:15437. [Crossref] [PubMed]
- Yu JB, Decker RH, Detterbeck FC, et al. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol 2010;5:215-9. [Crossref] [PubMed]
- Varlotto JM, Recht A, Flickinger JC, et al. Lobectomy leads to optimal survival in early-stage small cell lung cancer: a retrospective analysis. J Thorac Cardiovasc Surg 2011;142:538-46. [Crossref] [PubMed]
- de Hoyos A, DeCamp MM. Surgery for small cell lung cancer. Thorac Surg Clin 2014;24:399-409. [Crossref] [PubMed]
- Stamatis G. Neuroendocrine tumors of the lung: the role of surgery in small cell lung cancer. Thorac Surg Clin 2014;24:313-26. [Crossref] [PubMed]
- Rudin CM, Ismaila N, Hann CL, et al. Treatment of Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol 2015;33:4106-11. [Crossref] [PubMed]
- Péchoux CL, Sun A, Slotman BJ, et al. Prophylactic cranial irradiation for patients with lung cancer. Lancet Oncol 2016;17:e277-93. [Crossref] [PubMed]
- Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [Crossref] [PubMed]
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69-74. [Crossref] [PubMed]
- Bracci L, Moschella F, Sestili P, et al. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res 2007;13:644-53. [Crossref] [PubMed]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [Crossref] [PubMed]
- Reck M, Luft A, Szczesna A, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Ready N, Owonikoko TK, Postmus PE, et al. CheckMate 451: A randomized, double-blind, phase III trial of nivolumab (nivo), nivo plus ipilimumab (ipi), or placebo as maintenance therapy in patients (pts) with extensive-stage disease small cell lung cancer (ED-SCLC) after first-line platinum-based doublet chemotherapy (PT-DC). J Clin Oncol 2016;34:abstr TPS8579.
- Ott P, Felip E, Hiret S, et al. OA05.01 Pembrolizumab in Patients with Extensive-Stage Small Cell Lung Cancer: Updated Survival Results from KEYNOTE-028. J Thorac Oncol 2017;12:S259. [Crossref]
- Sequist LV, Chiang A, Gilbert J, et al. Clinical activity, safety and predictive biomarkers results from a phase Ia atezolizumab (atezo) trial in extensive-stage small cell lung cancer (ES-SCLC). Ann Oncol 2016;27:1425PD.
- Horn L, Reck M, Mok T, et al. PS01.57: IMpower133: a Phase I/III Study of 1L Atezolizumab with Carboplatin and Etoposide in Patients with Extensive-Stage SCLC: Topic: Medical Oncology. J Thorac Oncol 2016;11:S305-6. [Crossref] [PubMed]
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321-30. [Crossref] [PubMed]
- Amezquita RA, Kaech SM. Immunology: The chronicles of T-cell exhaustion. Nature 2017;543:190-1. [Crossref] [PubMed]
- Wherry EJ. T cell exhaustion. Nat Immunol 2011;12:492-9. [Crossref] [PubMed]
- Dong ZY, Zhong WZ, Zhang XC, et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res 2017;23:3012-24.
- Bouchard H, Viskov C, Garcia-Echeverria C. Antibody-drug conjugates-a new wave of cancer drugs. Bioorg Med Chem Lett 2014;24:5357-63. [Crossref] [PubMed]
- Augustyn A, Borromeo M, Wang T, et al. ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proc Natl Acad Sci U S A 2014;111:14788-93. [Crossref] [PubMed]
- Jiang T, Collins BJ, Jin N, et al. Achaete-scute complex homologue 1 regulates tumor-initiating capacity in human small cell lung cancer. Cancer Res 2009;69:845-54. [Crossref] [PubMed]
- Geffers I, Serth K, Chapman G, et al. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. J Cell Biol 2007;178:465-76. [Crossref] [PubMed]
- Ball DW. Achaete-scute homolog-1 and Notch in lung neuroendocrine development and cancer. Cancer Lett 2004;204:159-69. [Crossref] [PubMed]
- Chapman G, Sparrow DB, Kremmer E, et al. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Hum Mol Genet 2011;20:905-16. [Crossref] [PubMed]
- Meder L, Konig K, Ozretic L, et al. NOTCH, ASCL1, p53 and RB alterations define an alternative pathway driving neuroendocrine and small cell lung carcinomas. Int J Cancer 2016;138:927-38. [Crossref] [PubMed]
- Dylla SJ. Toppling high-grade pulmonary neuroendocrine tumors with a DLL3-targeted trojan horse. Mol Cell Oncol 2016;3:e1101515 [Crossref] [PubMed]
- Saunders LR, Bankovich AJ, Anderson WC, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015;7:302ra136 [Crossref] [PubMed]
- Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 2017;18:42-51. [Crossref] [PubMed]
- Chu QS, Markman B, Leighl N, et al. A phase 1/2 trial of a monoclonal antibody targeting fucosyl GM1 in relapsed/refractory small cell lung cancer (SCLC): Safety and preliminary efficacy. Ann Oncol 2016;27:493-6. [Crossref]
- Bolanos-Garcia VM. Aurora kinases. Int J Biochem Cell Biol 2005;37:1572-7. [Crossref] [PubMed]
- Nikonova AS, Astsaturov I, Serebriiskii IG, et al. Aurora A kinase (AURKA) in normal and pathological cell division. Cell Mol Life Sci 2013;70:661-87. [Crossref] [PubMed]
- Lu Y, Liu Y, Jiang J, et al. Knocking down the expression of Aurora-A gene inhibits cell proliferation and induces G2/M phase arrest in human small cell lung cancer cells. Oncol Rep 2014;32:243-9. [PubMed]
- Melichar B, Adenis A, Lockhart AC, et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol 2015;16:395-405. [Crossref] [PubMed]
- Spigel DR, Greco FA, Rubin MS, et al. Phase II study of maintenance sunitinib following irinotecan and carboplatin as first-line treatment for patients with extensive-stage small-cell lung cancer. Lung Cancer 2012;77:359-64. [Crossref] [PubMed]
- Owonikoko TK, Dahlberg SE, Khan SA, et al. A phase 1 safety study of veliparib combined with cisplatin and etoposide in extensive stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E2511). Lung Cancer 2015;89:66-70. [Crossref] [PubMed]
- Reck M, Horn L, Novello S, et al. Phase II study of roniciclib in combination with cisplatin/etoposide or carboplatin/etoposide as first-line therapy in subjects with extensive-disease small cell lung cancer (ED-SCLC). Ann Oncol 2016;27:1426PD.


