A nonresponding small cell lung cancer combined with adenocarcinoma
Introduction
Combined small cell lung cancer (C-SCLC), defined as small cell lung cancer (SCLC) combined with an additional component that consists of any of the histological types of non-small cell lung cancer (NSCLC) (usually adenocarcinoma, squamous cell carcinoma, or large cell carcinoma and, less commonly, giant cell carcinoma or spindle cell) is considered to be a subset of SCLC (1,2). C-SCLC accounts for 28% of all SCLC cases in surgical specimens (1). However, most of the studies have shown that C-SCLC accounts for 1–3.2% because less than 5% of the patients with SCLC are detected with stage T1–2N0M0. Only the patients with stage T1–2N0M0 are eligible for surgery, and it is extremely difficult to diagnose C-SCLC through biopsy (3-5). This study aimed to report a case of limited-stage SCLC not responding to chemotherapy. The patient was diagnosed with SCLC combined adenocarcinoma and treated through surgical resection.
Case presentation
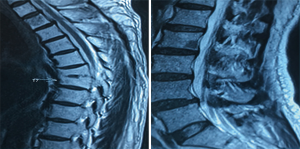
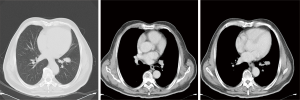
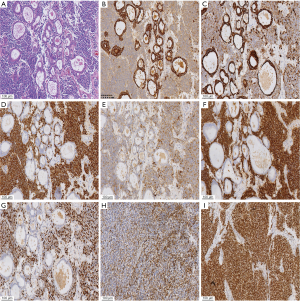
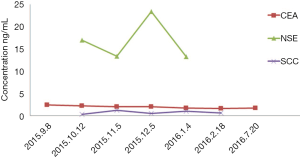
A 61-year-old heavy smoker in good physical condition was diagnosed with a mass in the left lung during a health examination through computed tomography (CT) scan. Fludeoxyglucose-positron emission tomography scan showed a mass in the left lower lung with hilar lymph nodes and no distant or cerebral metastasis. Bronchoscopy examination revealed SCLC. Two cycles of chemotherapy were given (etoposide combined with carboplatin/cisplatin), and stable disease was achieved (Figure 1). A radical R0 resection of left lower pulmonary carcinoma was performed, and the pathological diagnosis was SCLC combined adenocarcinoma (tumor 2.5 cm × 2 cm × 1.8 cm). The positive lymph nodes were left lower paratracheal nodes 0/6, aortopulmonary nodes 0/5, subcarinal nodes 0/4, paraesophageal nodes 0/1, hilar nodes 1/9, interlobar nodes 4/5, lobar nodes 0/3 and pTNM stage was pT1cN1M0 (IIB) according to the eighth edition of the TNM classification for lung cancer. The results of immunohistochemistry (IHC) analysis were as follows: CK/LMW (+), Ck8/18 (+), Syn (+), chromogranin A (+), CD56 (+), Ki-67 (+, 90%), P40 (−), P63(−), ALK (D5F3) (−), ALK-NC (−), Ck5/6 (−), CK7 (+), TTF1 (+), and napsin A (−) (Figure 2). The epidermal growth factor receptor (EGFR) mutation was found to be negative using the amplification refractory mutation system. The patient received two cycles of adjuvant chemotherapy with etoposide/carboplatin. Prophylactic radiotherapy was given in the mediastinal lymphatic drainage region, and the dose was as follows: hilum 5,000 cGy/25F, upper mediastinum 4,000 cGy/25 F, and spinal cord 3,000 cGy. One cycle of pemetrexed combined with carboplatin was given before radiotherapy. About 4 months after completing the treatment, bone metastasis occurred accompanied with back pain (Figure 3). Figure 4 shows the change in neuron-specific enolase (NSE), carcinoembryonic antigen (CEA), and squamous cell carcinoma (SCC) antigen. The sequence of anticancer treatments is shown in Table 1.
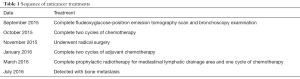
Full table
Discussion
SCLC is sensitive to first-line chemotherapy and radiotherapy, and lack of response to chemotherapy may be due to NSCLC complements (6,7). The tumors in patients with C-SCLC are easily located in the peripheral portion of the lung (3,8). The serum CEA level is invariably elevated in lung adenocarcinoma (9,10). The serum CEA level can be used as a reference marker in the diagnosis of SCLC combined adenocarcinoma (11). This patient did not respond to the initial two cycles of chemotherapy and received surgery, which confirmed SCLC combined adenocarcinoma. If the primary lesion is in the peripheral portion of the lung, high levels of CEA are observed, and the patient doses not respond to chemotherapy, the possibility of a tumor with combined histology should be considered in patients with SCLC on the basis of limited biopsy material.
Surgery in selected patients with limited-stage SCLC was associated with improved survival outcomes (12). Babakoohi et al. identified 22 patients with C-SCLC and compared the findings with the results obtained from 406 patients with pure SCLC. Surgery was significantly more common in patients with C-SCLC (2). Patients with C-SCLC carry a better prognosis than those with pure SCLC (2). The EGFR mutation is rare in patients with SCLC. It might occur more often in patients with C-SCLC, especially combined with adenocarcinoma, compared with patients with pure SCLCs (13-15). A few case reports showed that some patients with pure SCLC or C-SCLC having EGFR mutation respond to tyrosine kinase inhibitor (TKI) (15-17). It is thought that TKIs can also be used in C-SCLC with EGFR mutation, especially in second-line or third-line treatment. In this patient, no EGFR mutation was found.
Due to the difference of treatment and prognosis between C-SCLC and pure SCLC, further workup (e.g., repeat or multipoint biopsy) should be offered to reach an accurate pathological diagnosis and plan suitable treatment for those suspicious C-SCLC. Surgery should be considered for patients with suspicious early-stage C-SCLC. It can play an important role in diagnosis and treatment.
Acknowledgments
Funding: This study was funded by the Zhejiang Provincial Natural Science Foundation of China (No. LY15H290001), Public Welfare Technology Application Studies Program of Zhejiang Province (No. 2016C33118), and the 1022 Talent Training Program of Zhejiang Cancer Hospital.
Footnote
Conflicts of Interest: Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.06.16). The authors have no conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nicholson SA, Beasley MB, Brambilla E, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002;26:1184-97. [Crossref] [PubMed]
- Babakoohi S, Fu P, Yang M, et al. Combined SCLC clinical and pathologic characteristics. Clin Lung Cancer 2013;14:113-9. [Crossref] [PubMed]
- Mangum MD, Greco FA, Hainsworth JD, et al. Combined small-cell and non-small-cell lung cancer. J Clin Oncol 1989;7:607-12. [Crossref] [PubMed]
- Fraire AE, Johnson EH, Yesner R, et al. Prognostic significance of histopathologic subtype and stage in small cell lung cancer. Hum Pathol 1992;23:520-8. [Crossref] [PubMed]
- Rostad H, Naalsund A, Jacobsen R, et al. Small cell lung cancer in Norway. Should more patients have been offered surgical therapy? Eur J Cardiothorac Surg 2004;26:782-6. [Crossref] [PubMed]
- Shepherd FA, Ginsberg R, Patterson GA, et al. Is there ever a role for salvage operations in limited small-cell lung cancer? J Thorac Cardiovasc Surg 1991;101:196-200. [PubMed]
- Lad T, Piantadosi S, Thomas P, et al. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 1994;106:320S-323S. [Crossref] [PubMed]
- Fukui T, Tsuta K, Furuta K, et al. Epidermal growth factor receptor mutation status and clinicopathological features of combined small cell carcinoma with adenocarcinoma of the lung. Cancer Sci 2007;98:1714-9. [Crossref] [PubMed]
- Molina R, Filella X, Augé JM, et al. Tumor markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis. Comparison with the main clinical and pathological prognostic factors. Tumour Biol 2003;24:209-18. [Crossref] [PubMed]
- Wang CY, Huang MS, Huang MH, et al. Persistently high serum carcinoembryonic antigen levels after surgery indicate poor prognosis in patients with stage I non-small-cell lung cancer. J Surg Res 2010;163:e45-50. [Crossref] [PubMed]
- Lu HY, Mao WM, Cheng QY, et al. The effect of carcino-embryonic antigen and squamous cell carcinoma antigen in adjuvant diagnosis of conventional and combined small cell lung cancer. J Clin Oncol 2014;32:e18551
- Schreiber D, Rineer J, Weedon J, et al. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: should its role be re-evaluated? Cancer 2010;116:1350-7. [Crossref] [PubMed]
- Lu HY, Sun WY, Chen B, et al. Epidermal growth factor receptor mutations in small cell lung cancer patients who received surgical resection in China. Neoplasma 2012;59:100-4. [Crossref] [PubMed]
- Lu HY, Mao WM, Cheng QY, et al. Mutation status of epidermal growth factor receptor and clinical features of patients with combined small cell lung cancer who received surgical treatment. Oncol Lett 2012;3:1288-92. [PubMed]
- Tatematsu A, Shimizu J, Murakami Y, et al. Epidermal growth factor receptor mutations in small cell lung cancer. Clin Cancer Res 2008;14:6092-6. [Crossref] [PubMed]
- Okamoto I, Araki J, Suto R, et al. EGFR mutation in gefitinib-responsive small-cell lung cancer. Ann Oncol 2006;17:1028-9. [Crossref] [PubMed]
- Zakowski MF, Ladanyi M, Kris MG, et al. EGFR mutations in small-cell lung cancers in patients who have never smoked. N Engl J Med 2006;355:213-5. [Crossref] [PubMed]