
Autophagy regulation in bladder cancer as the novel therapeutic strategy
Introduction
Bladder cancer (BC) is the ninth most common cancer in Taiwanese male according to the Taiwan Cancer Registry Annual Report in 2014 (1). In the United State, BC is the sixth most commonly diagnosed cancer with estimated 79,030 new cases and 16,870 death in 2017 (2). The incidence and mortality rate of BC is just next to prostate cancer among the genitourinary malignancies. Almost 70–80% of patients with bladder tumors present with low-grade, superficial or non-muscle invasive bladder cancer (NMIBC) (3), others are muscle invasive bladder cancer (MIBC). Routine surveillance, repeated transurethral resection of bladder tumor (TUR-BT), and the use of intravesical agents are the standard procedures performed for initial diagnosis, staging and treatment for managing of NMIBC. Despite these efforts, there are still subset of patients who progress to MIBC that drive the mortality of these disease. While new treatment regiments are developed rapidly to manage other cancers, the treatment options for BC remains limited. It is notable that the 50% overall survival at 5 years for MIBC has not improved for almost 20 years. During the past decade, the one major change in management of BC was the introduction of neo adjuvant platinum-based chemotherapy to those patients suffering from non-metastatic MIBC. However, the overall mortality of BC has not changed. Therefore, development of novel therapeutic strategies or improvement of response rate to current therapies are critical to treat BC.
Autophagy and cancer
In 2016, the Nobel Prize in Physiology or Medicine was award to Prof. Yishinori Ohsumi for his discoveries of mechanisms of autophagy (4). Macroautophagy (autophagy, which means “self-eating”) is a highly conserved catabolic process that degrades cellular organelles and protein to maintain the cellular biosynthesis during nutrient deprivation or metabolic stress (5). The process of autophagy includes six steps: induction, initiation, elongation, maturation, fusion and degradation (Figure 1). These steps involved the formation of double-membranes that extent and engulf cytoplasmic constituents including organelles and protein to from vesicles (also known as autophagosomes) that subsequently fuse with lysosomes (as autophagolysosomes or autolysosomes), where the contents undergo degradation by the enzymes within the lysosome and recycling (5). Sustained, constitutive or basal autophagic activity is also important for all cells to remove damaged organelles as well as long-lived or misfolded proteins. Although autophagy was first observed as a protective mechanism in cells under starvation or metabolic pressures such as nutrition deprivation, hypoxia, ER stress, and chemotherapeutic agents (6), it has been demonstrated to play important roles during development and in numerous diseases, including infections, neurodegenerative and cardiovascular disease (7). Autophagy defects are known to associated with metabolic stress, genomic damage, and tumorigenesis in preclinical models, indicating its role in tumor suppression (8). Studies also showed that in 40–70% of human breast, prostate and ovarian cancers contain monoallelic loss of beclin-1, an essential autophagy gene, indicating that autophagy may be important in preventing these tumors. On the other hand, autophagic activity is related to the ability for the clearance of damaged organelles and misfolded protein, it also confers stress tolerance of tumor cells that usually under high metabolic rate associated with rapid cell proliferation to survive under adverse conditions. For example, autophagy is induced within hypoxic tumor regions of tumor cells (9). Stress induced autophagy in tumor cells could therefore resulted in tumor dormancy, drug resistant, and subsequently leading to tumor regrowth and procession. Accumulating studies had demonstrated that cancer cells induced autophagy to counteract with anticancer treatments by helping cells to evade apoptotic pathway (10). Therefore, many studies demonstrated that coordinated administration of autophagy inhibitors with chemotherapeutic agents suppressed tumor growth and resulted in a greater extent of cell death compared to chemotherapy alone (11). In the cellular or animal models, inhibition of pro-survival autophagy using pharmacological or genetic inhibitors was demonstrated to enhance apoptotic cell death and increased anti-cancer efficacy (12-16). These results reveal that targeting the prosurvival (or protective) autophagy in tumor cells may represent a novel strategy to develop successful cancer treatment. However, autophagy may also play dual roles (or referred as double-edged sword) in certain cellular contexts. It has been demonstrated that autophagic cell death was occurred in tumor cells, particularly in apoptosis-defective cells, that exhibit excessive or sustained autophagy (17). Thus, it is critical to understand the role of autophagy in cancer treatment as well as the elucidating the mechanisms involved in autophagy induction that influences tumorigenesis and treatment response. Analyzing the signaling pathway involved in autophagy induction may provide and identify new therapeutic targets for developing novel treatments that improve the outcomes.
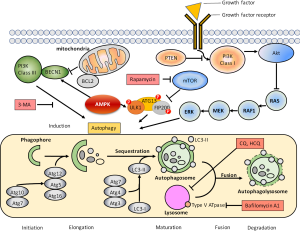
The molecular mechanism of autophagy regulation involves mammalian target of rapamycin (mTOR), 5'-AMP-activated protein kinase (AMPK), and the extracellular-signal-regulated kinase (ERK) (18). mTOR kinase that governing the expression of cellular protein is the major regulator to inhibit autophagy in the presence of growth factors or nutrition-rich conditions, while AMPK control autophagy in response to low energy or nutrient deprivation (19). Autophagy achieved by multiple processes and many autophagy-related genes that either participates in de novo membrane formation, autophagosomes formation, and fusion of lysosomes to autophagosomes for degradation or reuse of engulfed macromolecules (20). In our previous studies, we demonstrated that inhibition of autophagy is critical to improve the efficacy of RAD001, a mTOR inhibitor, in treating human BC cells (16). We also found that human bladder tumor exhibits high basal level of autophagy (15). It is possible that the high level of autophagy limits the effects of cisplatin treatment in BC cells, therefore, combination of chemotherapeutic regiment are required for treating BC. Recent studies indicated that cisplatin treatment induces protective autophagy in esophageal, lung and ovarian cancer cells (21-23). Hence, understanding the role of autophagy in BC progression and drug-resistant is important for designing novel strategies to improve current therapies.
The role of autophagy in BC progression
As mentioned above, most of the patients (~80%) present NMIBC at their first diagnosis. NMIBC has a high recurrence rate, and despite all the medical interventions, approximately 10-30% patients progress to MIBC (24). However, the exact mechanism of how NMIBC progression to MIBC is not fully understand. Several recent studies have shown the role of autophagy in BC progression. In 2013, Sivridis and his colleagues investigated the autophagy activity in BC tissues from 210 TUR specimens by detection of the expression pattern of microtubule-associated protein LC3A by immunohistochemical staining for its relevance with muscle invasion (25). They reported the detection of a grade-dependent increased number of “Stone like” structures (SLS) within cytoplasmic vacuoles under light microscopy. In this study, LC3A reactivity and the number of SLS were higher in high grade MIBC then in high grade NMIBC, suggesting that autophagy activity may also contribute to the progression of NMIBC to MIBC.
In 2013, Baspinar and his colleagues found a significant inverse correlation between the expression of beclin-1, an important mediator protein during autophagy initiation, and pT stages of BC using 84 tumor samples and 10 non-tumoral bladder tissues (26). They also identified that the expression level of Bcl-2, an antiapoptotic protein, correlated with histological grade. They concluded that Bcl-2 overexpression and down-regulation of beclin-1 play an important role in the progression and aggressiveness of BC. Like the proapoptotic proteins, beclin-1 contains a BH3 domain which is necessary and sufficient for binding to antiapoptotic Bcl-2 homologs (27). Therefore, interaction between beclin-1 and Bcl-2 is suggested to be a key switch mediating the crosstalk between the autophagy and apoptosis. According to the data from Baspinar et al., BC progression is correlated to the antiapoptotic properties by enhancing Bcl-2 expression but suppressing beclin-1. Another report by Liu et al. support this finding that beclin-1 is down-regulated in both mRNA and protein level during the progression of BC (28). However, whether down-regulation of beclin-1 indicating the inhibition of autophagy during the progression of BC is still inconclusive. More recent work by Wang et al. demonstrated the knockout and knockdown of retinoblastoma (Rb), a well-known tumor suppressor, resulted in autophagy and apoptosis inhibition via suppressing p53 and caspase-3 signaling, enhancing BC development in vitro and in vivo (29), supporting the finding that suppression of autophagy and apoptosis may be critical in BC progression.
In 2014, a group in India first published a paper demonstrating an increased grade-dependent autophagy in BC (30). They collected 15 high-grade and 15 low-grade tumor tissue samples, according to WHO criteria, from patients undergoing TUR-BT of NMIBC. Using transmission electron microscopy, they found a significant increase of autophagic vesicles in the high-grade specimens than in the low-grade specimens when compared with the benign tissues which obtained from patients undergoing TUR of prostate for benign prostatic hyperplasia. The expression levels of LC3-II protein, usually used as a marker for autophagic induction, and ATG7 and Beclin-1, key proteins involved in autophagosomes biogenesis, were increased in a grade-dependent manner in high- and low-grade BC tissues. To determine how cells from different grades of BC would respond to a common stress such as starvation, the primary tumor cells were grown under starvation for 0–48 h. The results showed that both high-grade and low-grade BC cells were more susceptible to starvation induced autophagy compared to normal urothelial cells. Furthermore, they identified that activation of AMPK signaling and inhibition of mTOR were involved in the induced autophagy under starvation in these BC cells. In addition, inhibition of starvation-induced autophagy using autophagy inhibitors, such as wortmannin, 3-methyladenine (3-MA), and chloroquine (CQ), increased cancer cell death also in a grade-dependent manner by triggering intrinsic apoptotic pathway.
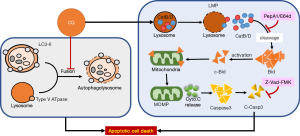
In 2016, we demonstrated that BC exhibits high basal level of autophagy by showing the increased LC3B expression levels in BC tissues from NMIBC patients compared with their paired adjacent normal tissues (15). Using well established BC cell lines, the basal autophagy activity that detected by the processing of LC3-II and LC3-positive puncta within the cytoplasm, was elevated in a grade-dependent manner in BC cells when compared to immortalized normal urothelial cells, or other reference cell lines including breast, prostate, and kidney cancer cells. Inhibition of basal autophagy using bafilomycin A1 (Baf A1, a specific inhibitor of vacuolar-type H(+)-ATPase which blocks the fusion of autophagosomes to lysosomes) or small hairpin RNA (shRNA) targeting ATG-7 decreased cancer cell viability through apoptosis induction. Our data demonstrated that BC tissues exhibits high basal level of autophagy that leads to two major conclusions: First, targeting basal autophagy may represent a novel therapeutic approach to treat BC. Second, the high basal autophagy is likely contributing to the drug resistant in BC against current chemotherapeutic agents. Since the growth of BC cells is depend on the highly activated basal autophagy, we therefore tried to treat the cancer cells using autophagy inhibitors alone and see if inhibition of basal autophagy decreased the cell viability efficiently. To our surprise, single treatment of CQ or HCQ significantly decreased cell viability via enhanced apoptosis in BC cells (31). The use of autophagy inhibitors exhibits severe cytotoxicity compared to standard chemotherapeutic agents such as Everolimus (RAD001) or cisplatin (16). To further explore the mechanism related to these autophagy inhibitors against BC cell, we investigated the apoptosis induction pathways in CQ-treated BC cells. The results demonstrated that CQ not only disrupted the autophagic flux by inhibiting the fusion of autophagosomes to lysosomes, but it targeted lysosomal functions to amplify apoptotic signals and ultimately leading to cancer cell death (Figure 2) (31). The metabolic activities of tumor cells function primarily to support the unusually high rates of cell growth and proliferation. Therefore, it is possible that high basal level of autophagy and lysosomal function are critical to support the growth and survival of BC cells. Targeting autophagy or lysosomal function may present a novel therapeutic approach for treating BC.
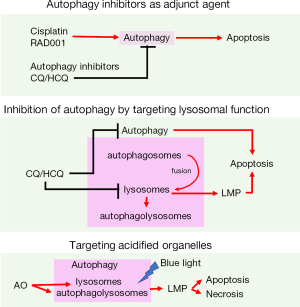
We also developed another potential therapeutic approach by taking advantages of high basal autophagy in the BC cells. Acridine orange (AO) vital staining is a conventional approach to detect acidic vesicles upon induction of autophagy. AO shows green fluorescence when bound to DNA and red when bound to acidic regions which includes lysosomes and autophagolysosomes. Increased number of AO-positive red puncta could serve as an autophagy indicator when studying autophagy induction in vitro. However, we constantly failed to detect autophagy induction using AO staining in BC cells, even when the detection of LC3-II progression suggests that autophagy was induced. By a closing look at the fluorescent pattern in AO-treated BC cells, we found a phenomenon which is called photooxidative damage that targeting lysosomes to enhance cytotoxicity effect of AO against BC cells. We discovered that AO treatment significantly reduced cell viability of BC cells under blue light exposure while had small impact on immortalized urothelial cells; because cancer cells contain increased number of acidified organelles including autophagolysosomes and lysosomes (32). This type of treatment was designated as AO photodynamic treatment (AO-PDT), and has the potential in further clinical application since current endoscopic light technology that urologist commonly utilized in TUR-BT could be equipped with blue light source. Strategies targeting autophagy to improve BC treatment in our research are illustrated in Figure 3. Our recent studies regarding the role of autophagy in urological cancers, including prostate and BC, are listed in Table 1.

Full table
As described above, the role of autophagy during BC progression is still controversial. However, based on these studies, autophagy becomes an important therapeutic target for it clearly plays a role in BC progression. Inhibition of autophagy activated during BC progression is likely to improve the responses to current therapies.
Coordinate autophagy inhibition with current approved therapies in BC treatment
Intravesical instillation
Standard treatment for NMIBC consists of complete TURBT of all visible lesions. Intravesical therapies followed by TURBT seems to reduce the recurrence rate to 25–50% in 2 years of follow-up, and is the current armamentarium for the management of NMIBC. Current intravesical instillation are classified as intravesical chemotherapy and immunotherapy, using agents such as mitomycin C (MM) and the bacillus Calmette-Guerin (BCG), respectively.
MM inhibits DNA synthesis is a chemotherapeutic agent that decreases the recurrence rate of NMIBC from 54% to 38% with no impact on the risk of progression (35). It has been demonstrated that other intravesical chemotherapeutic agents including doxorubicin, epirubicin and thiotepa are utilized for intravesical therapy and found no superiority of one drug over the others (36). Cancer stem cells (CSC) has been suggested to help tumor progression, and in some cases evade chemotherapeutics (37). Ojha’s group reported the isolation of side population (SP) cells which share characteristics of CSCs from BC cell lines (38). These SP cells showed substantial resistance to MM treatment, and exhibited higher autophagic flux. Inhibition of autophagy by CQ or siRNA against beclin-1 potentiated the chemotherapeutic effects of MM in these SP cells. In a followed-up study, they showed that MM treatment increased the percentage of CSCs in primary cultured urothelial carcinoma cells (39). These CSCs exhibits high level of autophagy. Inhibition of autophagy by CQ decreased the expression of drug resistance genes (MDR1 and ABCG2) and enhanced MM induced apoptosis. Therefore, synergistic cytotoxicity effect of MM with autophagy inhibitor may help to improve the outcome in NMIBC patients.
BCG, a live attenuated strain of Mycobacterium bovis, is one of the germ that causes tuberculosis (TB) (40). It has been used to treat NMIBC for more than three decades and is currently the only agent approved by the US Food and Drug Administration for the therapy of NMIBC. Despite the long term clinical experiences with BCG, the exact mechanism of its therapeutic effect against BC is still largely unknown. It is suggested that BCG triggers an immune response that immune cells such as macrophages and lymphocytes move into the tissues, as a part of the inflammatory reaction, when the bacteria attach and absorb to the tumor cells. Study showed that BCG is markedly superior to MM in high-risk than in low risk patients (41), making it a standard of care in high-risk NMIBC to prevent recurrence and progression (42). The induction of autophagy has been shown to be a defense mechanism inhibiting BCG survival in macrophages (43), thus modulating the immune response to BCG treatment. Recently, Buffen et al. reported that inhibition of autophagy blocked trained immunity induced by BCG (44). They also found that polymorphisms (SNPs) in the autophagy gene ATG2B and ATG5 negatively influence trained immunity in monocytes. These studies demonstrate the role of autophagy in successful BCG treatment, and point out future directions for improving BCG therapy.
Radical cystectomy is the standard of care following BCG failure (45). As an alternative therapy, valrubicin is the only agent approved in the US for BCG refractory (46). Other intravesical agents, while not recommended for primary treatment, have been studied for the treatment of NMIBC. The guidelines do allow for the consideration of alternative treatment of NMIBC in the setting of BCG failure. It has been demonstrated that salvage intravesical therapy using interferon in combination with BCG is effective (47). Zhang et al. reported that adenoviral-mediated interferon alpha treatment induces autophagy in BC, and inhibition of induced autophagy using 3-MA increases cytotoxicity (48). In other studies, promising results were reported using intravesical gemcitabine alone or in combination with MM in patients with BCG failure (49). The study by Ojha et al. using CQ as an autophagy inhibitor demonstrated similar effects as MM on a gemcitabine treated cell lines that reduces the expression of drug resistance genes (39). Addition of CQ also sensitized the CSCs to gemcitabine induced apoptosis, providing evidence that autophagy inhibition could be synergistic to the combination of gemcitabine/MM for BCG failure patients. A recent study by Amantini et al. demonstrated that capsaicin (CPS) triggers autophagy which drives epithelial mesenchymal transition (EMT) and chemoresistance in BC cells (50). CPS is the active alkaloid found primarily in the chili peppers and used as an intravesical drug for overactive bladder. They showed that the CPS-resistant EMP-positive BC cells displayed an increased drug-resistance to MM, gemcitabine, and doxorubicine which commonly used in BC therapy. Another study was conducted by Pan et al. (51) to investigate the effect and mechanisms of icaritin, a hydrolytic form of icariin which is one of the traditional Chinese herbals, against human BC cells. Although the exact mechanism is still unclear, they found that icaritin not only suppressed the basal autophagy, but inhibited epirubicin induced autophagy. Therefore, icaritin act synergistically with epirubicin to suppress the proliferation of BC cells. Induction of autophagy had been reported using another intravesical chemotherapy agent, pirarubicin, for BC (52). In this study, Li et al. showed that pirarubicin-induced autophagy was mediated via mTOR signaling pathway; and inhibition of induced autophagy using siRNA against ATG3, 3-MA, or hydroxychloroquine (HCQ) significantly induced apoptosis. These studies further supported the idea that autophagy activities in BC is related to its progression and drug resistance.
Chemotherapy
Approximately 30% of newly diagnosed BC patients present with MIBC, and as much as 30% of patients with NMIBC eventually progress to muscle-invasion. Surgery, specifically radical cystectomy is the standard procedure in treating MIBC (53). A major change in treatment of MIBC is the development of platinum-based chemotherapies which are a standard of care (54,55). The two regimens of neoadjuvant chemotherapy are either methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) or gemcitabine and cisplatin (GC). As mentioned above, autophagy is considered as a survival mechanism in cancer cells to evade apoptosis against chemotherapeutic agents. Therefore, a significant amount of studies focus on the potential relationship of autophagy induced by these drugs. CQ, the antimalarial drug and a well-known autophagy inhibitor, enhanced cisplatin mediated cytotoxicity in BC cells without affecting normal urothelial cells (30). Administration of 3-MA, a class III phosphoinositide 3-kinase (PI3K) inhibitor, known to inhibit early stage of autophagy processing enhanced the cytotoxicity of (–)-gossypol, a pan Bcl-2 inhibitor, in cisplatin-resistant BC cell lines (56). Everolimus (RAD001), a specific inhibitor of mTORC1 complex, is used for the treatment of metastatic renal cell carcinoma, but is not effective in the treatment of BC (57). Inhibition of mTOR is known to induce autophagy in cells under starvation (58). Therefore, it is possible that chemotherapeutic agents targeting mTOR induce autophagy which promotes tumor survival, and thus these agents potentially limit their own efficacy. As a prove of principle study, we demonstrated that inhibition of RAD001-induced autophagy using inhibitors, including 3-MA, Baf A1, CQ, or HCQ, significantly decreased the cell viability by enhancing apoptosis of BC cell lines (16). In consist with our findings (59), Fan et al. demonstrated that cisplatin treatment induced significant autophagy in BC T24 cell line (60). They reported that cisplatin induces autophagy through inhibition of mTOR pathway, and targeting ATG8 (LC3) significantly reduced the cell viability in cisplatin-treated cells. Recently, Ojha et al. took this one step further (61). They established cisplatin resistant patient derived primary culture cells and treated these cells with gemcitabine and MM. The results showed that resistant cells have higher basal autophagic flux. Gemcitabine and MM further induced autophagy in cisplatin-resistant cells; and combination of autophagy inhibitors (CQ or knockdown of beclin-1) synergistically inhibited BC cell growth. They also reported that INF-γ mediated JAK2 pathway is responsible for the autophagy-related drug resistant in BC.
MicroRNAs (miRNAs) are small noncoding RNAs (~20–24 nucleotides) that regulates their targeted gene expression through translational blockage or mRNA degradation (62). To date, there are 2,588 matured human miRNAs registered in the miRNA database (miRbase, release 21; http://www.mirbase.org). Increasing studies have shown that miRNAs play important roles that regulate tumor formation and progression (63). The important role of dysregulated miRNAs in tumorogenesis has emerged as a new field in cancer research. In BC, profiling of miRNA expression patterns by large scale microarray approaches started at 2007 (64). After the first publication, 7 studies were published in different countries (65-71). And only a few number of validated miRNA target genes were found. Using a microarray approach, we also showed that 11 and 19 miRNAs were up-regulated and down-regulated, respectively, in human BC tissues (72). Among these dysregulated miRNAs, the miR-99 family which are down-regulated was found to target the mTOR signaling pathway in other cancers (73-76). In addition, miR-30a which also found to be down-regulated in BC, was demonstrated to mediated autophagy inhibition that sensitizes renal cell carcinoma cells to sorafenib (77). Although further studies are needed, these miRNAs may involve in the regulation of autophagy in BC. On the other hand, miR-222 that up-regulated in BC was found to be associated with a poor prognosis in BC (78). Zeng et al. demonstrated that overexpression of miR-222 activated the Akt/mTOR pathway and inhibited cisplatin-induced autophagy by directly targeting protein phosphatase 2A subunit B (PPP2R2A) (79). Therefore, miR-222 may present a viable future target for attenuating cisplatin-resistance. More recently, the up-regulation of miR-24-3p was reported in BC (80). In this study, miR-24-3p was found to induce autophagy by suppressing the expression of DEDD, a member of the death effector domain-containing protein family. However, the role of miR-24-3p induced autophagy and its association with drug-resistance remained further investigation.
These studies involving the administration of autophagy inhibitors, including pharmaceutical drugs, such as CQ and HCQ, or molecular inhibitors, such as siRNAs/miRNAs targeting autophagic proteins, demonstrate the further research targeting autophagy process can not only improve the response to current therapies but provide new strategies and targets for future development of novel therapies against BC.
Conclusions
As a major protective mechanism, autophagy is now recognized to play a key role in the stress response against various conditions such as nutrient starvation, hypoxia, infection, and tumor development. There are several on-going clinical trials using autophagy-modulating compounds alone or combined with conventional anti-cancer drugs to treat various types of cancer, including prostate, renal, lung, breast, pancreatic and kidney, but not BC (81). Furthermore, there is no breakthrough to the management of BC, despite its high morbidity in the past decades. The involvement of autophagy in the progression and in treatment resistance in BC is gaining more attraction. Targeting autophagy may help to increase the current therapies and develop novel therapeutic strategies against BC.
Acknowledgments
Funding: The study was supported by Shin Kong Wu Ho-Su Memorial Hospital (SKH-8302-103-0201, SKH-8302-104-0201, and SKH-8302-105-0201).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ja Hyeon Ku, Kunyoo Shin, Minyong Kang) for the series “Bladder Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.06.26). The series “Bladder Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Health Promotion Administration. Cancer Registry Annual Report, 2014 Taiwan. Taiwan: Ministry of Health and Welfare, 2016.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: non-muscle-invasive bladder cancer. BJU Int 2017;119:371-80. [Crossref] [PubMed]
Yoshinori Ohsumi - Facts - Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer 2007;7:961-7. [Crossref] [PubMed]
- Lum JJ, Bauer DE, Kong M, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 2005;120:237-48. [Crossref] [PubMed]
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med 2013;368:1845-6. [Crossref] [PubMed]
- Qu X, Yu J, Bhagat G, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest 2003;112:1809-20. [Crossref] [PubMed]
- Mazure NM, Pouyssegur J. Hypoxia-induced autophagy: cell death or cell survival? Curr Opin Cell Biol 2010;22:177-80. [Crossref] [PubMed]
- Kondo Y, Kanzawa T, Sawaya R, et al. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 2005;5:726-34. [Crossref] [PubMed]
- Yang ZJ, Chee CE, Huang S, et al. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther 2011;10:1533-41. [Crossref] [PubMed]
- Mulcahy Levy JM, Zahedi S, Griesinger AM, et al. Autophagy inhibition overcomes multiple mechanisms of resistance to BRAF inhibition in brain tumors. Elife 2017;6:e19671 [Crossref] [PubMed]
- Kang M, Lee KH, Lee HS, et al. Concurrent Autophagy Inhibition Overcomes the Resistance of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Human Bladder Cancer Cells. Int J Mol Sci 2017;18:E321 [Crossref] [PubMed]
- Hou W, Song L, Zhao Y, et al. Inhibition of Beclin-1-Mediated Autophagy by MicroRNA-17-5p Enhanced the Radiosensitivity of Glioma Cells. Oncol Res 2017;25:43-53. [Crossref] [PubMed]
- Lin YC, Lin JF, Wen SI, et al. Inhibition of High Basal Level of Autophagy Induces Apoptosis in Human Bladder Cancer Cells. J Urol 2016;195:1126-35. [Crossref] [PubMed]
- Lin JF, Lin YC, Yang SC, et al. Autophagy inhibition enhances RAD001-induced cytotoxicity in human bladder cancer cells. Drug Des Devel Ther 2016;10:1501-13. [Crossref] [PubMed]
- Maiuri MC, Zalckvar E, Kimchi A, et al. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 2007;8:741-52. [Crossref] [PubMed]
- Klionsky DJ. Coming soon to a journal near you - the updated guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2014;10:1691. [Crossref] [PubMed]
- Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 2007;8:931-7. [Crossref] [PubMed]
- Mizushima N. Autophagy: process and function. Genes Dev 2007;21:2861-73. [Crossref] [PubMed]
- O'Donovan TR, O'Sullivan GC, McKenna SL. Induction of autophagy by drug-resistant esophageal cancer cells promotes their survival and recovery following treatment with chemotherapeutics. Autophagy 2011;7:509-24. [Crossref] [PubMed]
- Sirichanchuen B, Pengsuparp T, Chanvorachote P. Long-term cisplatin exposure impairs autophagy and causes cisplatin resistance in human lung cancer cells. Mol Cell Biochem 2012;364:11-8. [Crossref] [PubMed]
- Wang J, Wu GS. Role of autophagy in cisplatin resistance in ovarian cancer cells. J Biol Chem 2014;289:17163-73. [Crossref] [PubMed]
- van Rhijn BW, Burger M, Lotan Y, et al. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol 2009;56:430-42. [Crossref] [PubMed]
- Sivridis E, Koukourakis MI, Mendrinos SE, et al. Patterns of autophagy in urothelial cell carcinomas--the significance of "stone-like" structures (SLS) in transurethral resection biopsies. Urol Oncol 2013;31:1254-60. [Crossref] [PubMed]
- Baspinar S, Bircan S, Yavuz G, et al. Beclin 1 and bcl-2 expressions in bladder urothelial tumors and their association with clinicopathological parameters. Pathol Res Pract 2013;209:418-23. [Crossref] [PubMed]
- Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene 2008;27:S137-48. [Crossref] [PubMed]
- Liu GH, Zhong Q, Ye YL, et al. Expression of beclin 1 in bladder cancer and its clinical significance. Int J Biol Markers 2013;28:56-62. [Crossref] [PubMed]
- Wang CY, Xu ZB, Wang JP, et al. Rb deficiency accelerates progression of carcinoma of the urinary bladder in vivo and in vitro through inhibiting autophagy and apoptosis. Int J Oncol 2017;50:1221-32. [PubMed]
- Ojha R, Singh SK, Bhattacharyya S, et al. Inhibition of grade dependent autophagy in urothelial carcinoma increases cell death under nutritional limiting condition and potentiates the cytotoxicity of chemotherapeutic agent. J Urol 2014;191:1889-98. [Crossref] [PubMed]
- Lin YC, Lin JF, Wen SI, et al. Chloroquine and hydroxychloroquine inhibit bladder cancer cell growth by targeting basal autophagy and enhancing apoptosis. Kaohsiung J Med Sci 2017;33:215-23. [Crossref] [PubMed]
- Lin YC, Lin JF, Tsai TF, et al. Acridine orange exhibits photodamage in human bladder cancer cells under blue light exposure. Scitific Report (Revised) 2017.
- Lin JF, Lin YC, Lin YH, et al. Zoledronic acid induces autophagic cell death in human prostate cancer cells. J Urol 2011;185:1490-6. [Crossref] [PubMed]
- Lin JF, Tsai TF, Liao PC, et al. Benzyl isothiocyanate induces protective autophagy in human prostate cancer cells via inhibition of mTOR signaling. Carcinogenesis 2013;34:406-14. [Crossref] [PubMed]
- Shariat SF, Chade DC, Karakiewicz PI, et al. Update on intravesical agents for non-muscle-invasive bladder cancer. Immunotherapy 2010;2:381-92. [Crossref] [PubMed]
- Abern MR, Owusu RA, Anderson MR, et al. Perioperative intravesical chemotherapy in non-muscle-invasive bladder cancer: a systematic review and meta-analysis. J Natl Compr Canc Netw 2013;11:477-84. [Crossref] [PubMed]
- Stuckey DW, Shah K. Stem cell-based therapies for cancer treatment: separating hope from hype. Nat Rev Cancer 2014;14:683-91. [Crossref] [PubMed]
- Ojha R, Jha V, Singh SK, et al. Autophagy inhibition suppresses the tumorigenic potential of cancer stem cell enriched side population in bladder cancer. Biochim Biophys Acta 2014;1842:2073-86.
- Ojha R, Jha V, Singh SK. Gemcitabine and mitomycin induced autophagy regulates cancer stem cell pool in urothelial carcinoma cells. Biochim Biophys Acta 2016;1863:347-59.
- Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nat Rev Urol 2014;11:153-62. [Crossref] [PubMed]
- Shelley MD, Court JB, Kynaston H, et al. Intravesical bacillus Calmette-Guerin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Rev 2003;CD003231 [PubMed]
- Gandhi NM, Morales A, Lamm DL. Bacillus Calmette-Guerin immunotherapy for genitourinary cancer. BJU Int 2013;112:288-97. [Crossref] [PubMed]
- Gutierrez MG, Master SS, Singh SB, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004;119:753-66. [Crossref] [PubMed]
- Buffen K, Oosting M, Quintin J, et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog 2014;10:e1004485 [Crossref] [PubMed]
- Power NE, Izawa J. Comparison of Guidelines on Non-Muscle Invasive Bladder Cancer (EAU, CUA, AUA, NCCN, NICE). Bladder Cancer 2016;2:27-36. [Crossref] [PubMed]
- Steinberg G, Bahnson R, Brosman S, et al. Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol 2000;163:761-7. [Crossref] [PubMed]
- O'Donnell MA, Krohn J, DeWolf WC. Salvage intravesical therapy with interferon-alpha 2b plus low dose bacillus Calmette-Guerin is effective in patients with superficial bladder cancer in whom bacillus Calmette-Guerin alone previously failed. J Urol 2001;166:1300-4, discussion 4-5. [Crossref] [PubMed]
- Zhang XQ, Dunner K Jr, Benedict WF. Autophagy is induced by adenoviral-mediated interferon alpha treatment in interferon resistant bladder cancer and normal urothelial cells as a cell death protective mechanism but not by the bystander factors produced. Cancer Gene Ther 2010;17:579-84. [Crossref] [PubMed]
- Cockerill PA, Knoedler JJ, Frank I, et al. Intravesical gemcitabine in combination with mitomycin C as salvage treatment in recurrent non-muscle-invasive bladder cancer. BJU Int 2016;117:456-62. [Crossref] [PubMed]
- Amantini C, Morelli MB, Nabissi M, et al. Capsaicin triggers autophagic cell survival which drives epithelial mesenchymal transition and chemoresistance in bladder cancer cells in an Hedgehog-dependent manner. Oncotarget 2016;7:50180-94. [PubMed]
- Pan XW, Li L, Huang Y, et al. Icaritin acts synergistically with epirubicin to suppress bladder cancer growth through inhibition of autophagy. Oncol Rep 2016;35:334-42. [PubMed]
- Li K, Chen X, Liu C, et al. Pirarubicin induces an autophagic cytoprotective response through suppression of the mammalian target of rapamycin signaling pathway in human bladder cancer cells. Biochem Biophys Res Commun 2015;460:380-5. [Crossref] [PubMed]
- Stenzl A, Cowan NC, De Santis M, et al. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol 2011;59:1009-18. [Crossref] [PubMed]
- Lázaro M, Gallardo E, Domenech M, et al. SEOM Clinical Guideline for treatment of muscle-invasive and metastatic urothelial bladder cancer (2016). Clin Transl Oncol 2016;18:1197-205. [Crossref] [PubMed]
- Chou R, Selph SS, Buckley DI, et al. Treatment of muscle-invasive bladder cancer: A systematic review. Cancer 2016;122:842-51. [Crossref] [PubMed]
- Mani J, Vallo S, Rakel S, et al. Chemoresistance is associated with increased cytoprotective autophagy and diminished apoptosis in bladder cancer cells treated with the BH3 mimetic (-)-Gossypol (AT-101). BMC Cancer 2015;15:224. [Crossref] [PubMed]
- Oudard S, Medioni J, Aylllon J, et al. Everolimus (RAD001): an mTOR inhibitor for the treatment of metastatic renal cell carcinoma. Expert Rev Anticancer Ther 2009;9:705-17. [Crossref] [PubMed]
- Zhao J, Goldberg AL. Coordinate regulation of autophagy and the ubiquitin proteasome system by MTOR. Autophagy 2016;12:1967-70. [Crossref] [PubMed]
- Hwang TI, Tsai TF, Yang SC, et al. Enhanced apoptosis by inhibition of cisplatin-induced autophagy in human bladder cancer cells. Urological Science 2016;27:S3. [Crossref]
- Fan Z, Huangfu X, Liu Z. Effect of autophagy on cisplatin-induced bladder cancer cell apoptosis. Panminerva Med 2017;59:1-8. [PubMed]
- Ojha R, Singh SK, Bhattacharyya S. JAK-mediated autophagy regulates stemness and cell survival in cisplatin resistant bladder cancer cells. Biochim Biophys Acta 2016;1860:2484-97.
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 2004;5:522-31. [Crossref] [PubMed]
- Iorio MV, Croce CM. Causes and consequences of microRNA dysregulation. Cancer J 2012;18:215-22. [Crossref] [PubMed]
- Gottardo F, Liu CG, Ferracin M, et al. Micro-RNA profiling in kidney and bladder cancers. Urol Oncol 2007;25:387-92. [Crossref] [PubMed]
- Wiklund ED, Bramsen JB, Hulf T, et al. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int J Cancer 2011;128:1327-34. [Crossref] [PubMed]
- Wang G, Zhang H, He H, et al. Up-regulation of microRNA in bladder tumor tissue is not common. Int Urol Nephrol 2010;42:95-102. [Crossref] [PubMed]
- Neely LA, Rieger-Christ KM, Neto BS, et al. A microRNA expression ratio defining the invasive phenotype in bladder tumors. Urol Oncol 2010;28:39-48. [Crossref] [PubMed]
- Wszolek MF, Rieger-Christ KM, Kenney PA, et al. A MicroRNA expression profile defining the invasive bladder tumor phenotype. Urol Oncol 2011;29:794-801.e1. [Crossref] [PubMed]
- Ichimi T, Enokida H, Okuno Y, et al. Identification of novel microRNA targets based on microRNA signatures in bladder cancer. Int J Cancer 2009;125:345-52. [Crossref] [PubMed]
- Catto JW, Miah S, Owen HC, et al. Distinct microRNA alterations characterize high- and low-grade bladder cancer. Cancer Res 2009;69:8472-81. [Crossref] [PubMed]
- Baffa R, Fassan M, Volinia S, et al. MicroRNA expression profiling of human metastatic cancers identifies cancer gene targets. J Pathol 2009;219:214-21. [Crossref] [PubMed]
- Tsai TF, Lin YC, Chen HE, et al. Involvement of the insulin-like growth factor I receptor and its downstream antiapoptotic signaling pathway is revealed by dysregulated microRNAs in bladder carcinoma. Urological Science 2014;25:58-64. [Crossref]
- Huang HG, Luo X, Wu S, et al. MiR-99a Inhibits Cell Proliferation and Tumorigenesis through Targeting mTOR in Human Anaplastic Thyroid Cancer. Asian Pac J Cancer Prev 2015;16:4937-44. [Crossref] [PubMed]
- Yang Z, Han Y, Cheng K, et al. miR-99a directly targets the mTOR signalling pathway in breast cancer side population cells. Cell Prolif 2014;47:587-95. [Crossref] [PubMed]
- Wang L, Chang L, Li Z, et al. miR-99a and -99b inhibit cervical cancer cell proliferation and invasion by targeting mTOR signaling pathway. Med Oncol 2014;31:934. [Crossref] [PubMed]
- Hu Y, Zhu Q, Tang L. MiR-99a antitumor activity in human breast cancer cells through targeting of mTOR expression. PLoS One 2014;9:e92099 [Crossref] [PubMed]
- Zheng B, Zhu H, Gu D, et al. MiRNA-30a-mediated autophagy inhibition sensitizes renal cell carcinoma cells to sorafenib. Biochem Biophys Res Commun 2015;459:234-9. [Crossref] [PubMed]
- Zhang DQ, Zhou CK, Jiang XW, et al. Increased expression of miR-222 is associated with poor prognosis in bladder cancer. World J Surg Oncol 2014;12:241. [Crossref] [PubMed]
- Zeng LP, Hu ZM, Li K, et al. miR-222 attenuates cisplatin-induced cell death by targeting the PPP2R2A/Akt/mTOR Axis in bladder cancer cells. J Cell Mol Med 2016;20:559-67. [Crossref] [PubMed]
- Yu G, Jia Z, Dou Z. miR-24-3p regulates bladder cancer cell proliferation, migration, invasion and autophagy by targeting DEDD. Oncol Rep 2017;37:1123-31. [PubMed]
- Jiang P, Mizushima N. Autophagy and human diseases. Cell Res 2014;24:69-79. [Crossref] [PubMed]


