
Increasing the power of tumour control and normal tissue complication probability modelling in radiotherapy: recent trends and current issues
Introduction
Biological models in radiotherapy (RT) began to appear in the late 1980s and early 1990s at the dawn of the ‘3D conformal era’ (1-3). Around the same time, the dose-volume histogram (DVH) concept entered the RT treatment-planning arena. The DVH represented a major simplification of the then ‘new’ 3D dose distributions in target and normal-tissue volumes; the potential of using these DVHs to estimate (local) tumour control probability (TCP) and normal tissue complication probability (NTCP) was soon recognized, and mathematical functions for TCP and NTCP began to appear in the literature (4).
By taking the DVH as their starting point, these early TCP and NTCP models effectively ignored possible regional differences in radiosensitivity within the structures of interest. Such models are a hybrid of the patient-specific (i.e., organ- and target-volume specific) DVH and a patient-population-based TCP or NTCP model. Thus the statement ‘for this patient the rectal NTCP =5%’ is to be interpreted as ‘for patients with precisely this rectal DVH, 5 out of 100 will suffer a complication’ (of a specific type e.g., grade 2 rectal bleeding); similarly, a TCP of 43% for a non-small-cell lung tumour means 43 patients out of 100 with that particular target DVH [say for the clinical target volume (CTV)] will be controlled locally. If the NTCP and TCP vs. prescription dose curves are computed from the mathematical expressions for TCP and NTCP then fairly shallow curves result, as opposed to a ‘binary’ situation (Figure 1): lower x-axis-(prescription) dose & no complication/tumour control; upper x-axis-(prescription) dose & certain complication/tumour control. Such models, provided their parameters are derived from best fits to DVH-based clinical outcomes, possess a logical consistency, at least within the range of doses over which the fitting was carried out. It is equally logical to suppose that the shallowness of the curves as a function of the dose is a result of a multiplicity of unknown factors. A clear example is the Marsden TCP model (5,6), which explicitly incorporates a spread in radiosensitivity over the population by considering a range of alpha values (i.e., sigma-alpha). Were the radiosensitivity of tumour clonogens for the individual patient’s tumour known exactly or at least within tighter limits, then a much steeper TCP vs. (tumour) dose curve would result, approaching in the limit a binary control/failure threshold, thereby yielding the dose that just achieves local control.
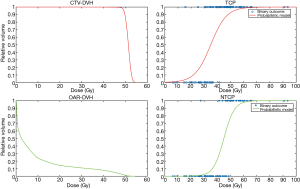
NTCP models reduce complex 3D dosimetric and anatomical information to a single risk measure. Modelling of NTCP has been performed with different techniques for many organs and endpoints. The Lyman-Kutcher-Burman (LKB) and relative seriality (RS) models (1,7), in particular, are the most well-known and traditionally accepted methods for predicting toxicity after radiation treatment. Conventional NTCP models are uniquely based on DVH and fractionation information and implicitly treat the organ in question as homogeneous in its response to radiation. Given their DVH-based nature, they cannot by definition handle differences in radiation response in different sub-volumes. Furthermore, such models (e.g., LKB, RS) are far less mechanistic in nature than the cell-killing TCP models. In addition, RT outcomes may also be affected by multiple factors other than dosimetric parameters; tissue response to radiation involves complex interactions of physical, biological and clinical factors (8). The identification of these additional factors is needed to improve the predictive power of the models.
It is important to emphasize that the ‘population’ nature of the DVH-based TCP and NTCP models in no way invalidates the concept of ‘isotoxic’ prescription-dose individualization (3,4,6,9,10). Quite the reverse—any increases in the specificity of these models (e.g., an increase in the slope) can only result in improved treatment individualization: “the baby must not be thrown out with the bathwater”. A model can be extremely useful while being far from perfect.
This review will focus on recent advances in the field of TCP and NTCP modelling, discussing possible strategies to go beyond the concept of a risk/benefit analysis based solely on DVH information, aiming at an increased personalization of RT treatments.
TCP models: biological targeting of the tumour
Most of the current TCP models start from the assumption that tumour control, i.e., the stopping of tumour growth, requires all clonogenic cells to be ‘killed’ by the radiation treatment. Poisson statistics are employed in the classical approach, combined with the so-called linear-quadratic (LQ) model, to calculate the probability of complete cancer-cell inactivation, i.e., reproductive death, as a function of radiation dose (6). Reviews have been published summarizing the mathematical formalisms of the most common TCP models (4-6). In order to enable TCP models to accurately predict clinical response, input parameters such as the intrinsic radiosensitivity of the clonogenic cells and their total number in the tumour are required. These parameters are usually unknown for individual tumours but estimates are available, mainly from in vitro studies (11,12). At the same time, there is a growing consensus in the RT community that inter- and intra-tumour heterogeneity represent important players for the radiation response of groups of human cancers. This heterogeneity arises from several aspects, including distributions in intrinsic tumour radiosensitivity, oxygenation status and growth fraction.
In this framework, because of the technical advances achieved in RT in the last decade, TCP models ought now to be included in the treatment-planning workflow. In parallel, progress in biomedical imaging has made it possible to obtain information on several of the sources of heterogeneity listed above, mainly by MRI- and PET-based techniques (13). This paves the way for the identification of what Ling et al. defined as ‘biological target volume’ (BTV, Figure 2) in their seminal paper (14). The BTV concept is based on the hypothesis that local relapses are likely to arise from niches of radioresistant cells, or from local concentrations of clonogens that are present in some regions at higher densities. If these sub-regions can be identified by means of non-invasive tools, treatment plans can be optimized accordingly. We thus move towards a scenario where TCP models and bio-imaging can be used in synergy in order to improve clinical outcomes.
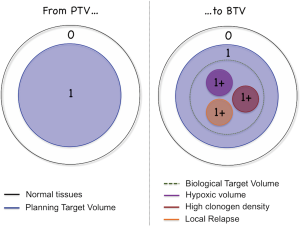
Image-guided dose escalation strategies
As summarized by Bentzen and Gregoire in a review on the incorporation of PET tracers in RT (15), including biological imaging in treatment planning constitutes a departure from the conventional concept of delivering a homogeneous dose to the target volume; instead it aims to take into account tumour heterogeneity. This can be done by ‘dose boosting’ or at the single-voxel level, so-called ‘dose painting by numbers’ (DPBN). Dose boosting requires a target defined from either quantitative or morphological aspects that enable the identification of one (or more) macroscopic region inside the PTV. The dose is then escalated only in a restricted volume. Nowadays dose boosting is in widespread clinical use, for instance for post-operative breast cancer (16) and for some head and neck lesions (17). In contrast, DPBN is still largely on the clinical practice ‘drawing board’. It is essentially based on the mathematical transformation of image intensity into voxel values according to the dose grid adopted. These image intensities contain biological information (e.g., oxygenation level, clonogen density, proliferation status) to be used to escalate the dose locally, prescribing doses voxel-by-voxel by inverse planning (18,19). In this context, TCP models are particularly important for quantifying the magnitude of the clinical gain compared to homogeneous irradiation. The studies presented in what follows include examples of both approaches.
Dose escalation based on tumour biopsy
Despite the debate on the usefulness of dose-escalation and of related techniques, there are not many papers that explicitly combine dosimetric analysis and TCP evaluation. Most of the relevant publications have investigated targeted dose escalation for prostate cancer. The goal is usually the targeting of the region of the tumour expected either to be more radioresistant, or to have a higher clonogen density, and therefore a higher risk of local failure (with homogeneous irradiation). Already in 2002, Nutting et al. presented a feasibility study combining biopsy information with CT imaging in order to identify the so-called dominant intra-prostatic tumour nodule (IPTN), which is a large nodule postulated to be responsible for local failures (20). Despite the fact that advanced imaging techniques for the prior definition of IPTNs were not available at the time, by using a patient-by-patient delta-TCP analysis (21) they were able to demonstrate that in principle an increase in TCP should be expected by delivering 70 Gy to the whole prostate and a 90 Gy boost to the dominant IPTN, with no significant increase in rectum toxicity. Remarkably, these results are also supported by recently published clinical investigations. In fact, even though a small number of patients was considered, the data published by Uzan et al. (22) and Onjukka et al. (23) suggest that dose boosting strategies based on MRI guidance are feasible and should be further investigated.
A more extensive TCP analysis is included in the work presented by Ghobadi et al. (24). Based on microscopy slides, Ghobadi and colleagues assigned a Gleason score (GS) to the different lesions derived from 25 patients. LQ TCP was calculated as the product of GTV and CTV-TCP, including also the information in terms of GS as a modification factor. This leads to different α values for the different lesions, thus reflecting differences in radiosensitivity corresponding to the heterogeneity of the lesion. Their results also show that taking into account heterogeneity results in a spread of TCP curves among patients, with their positions shifted along the dose axis according to the histopathology of the specific patient.
MRI and PET imaging: tools for BTV definition
Several in silico studies have been published, demonstrating the feasibility and the potential gain in TCP from a combination of CT with MRI (25,26) or PET (27-29) to define a BTV for the prostate, and the most recent of them will now be discussed. Casares-Magaz and colleagues presented a study where a Poisson-based TCP model was combined with MRI and apparent diffusion coefficient (ADC) maps in order to distribute the dose to the target according to the expected local cancer cell density (26). Their study aimed at reproducing the two arms of the FLAME trial (30): the standard arm receiving 77 Gy at 2.2 Gy/fraction to the PTV, the experimental arm including an additional boost region where dose is escalated up to 95 Gy at 2.6 Gy/fraction. The information carried by ADC maps obtained for 20 patients was converted into single-voxel cell densities using three different approaches (linear, binary and sigmoid relations). Dose was distributed according to the two trial arms, and TCP was then calculated combining the dose information with the cell density. Two important results were obtained: ADC map-based TCP predictions show greater inter-patient variation compared to the assumption of a uniform cell density; the mean TCP increased from 70% for the standard arm to about 95% for the experimental arm with the boost. The study showed that DPBN based on voxel-level cell-density information is feasible and is expected to yield higher TCP, thereby constituting an important step in the direction of personalized RT.
As mentioned above, PET has also been employed to identify the BTV in prostate cancer. Seppälä et al. presented in 2009 a feasibility study (27), and later Kuang and colleagues investigated boosting the dose to the PET-based BTV with volumetric modulated arc therapy (VMAT) (29). They considered 30 patients with localized prostate cancer who had undergone PET/CT before treatment. The 18F-choline PET tracer was employed, which shows increased uptake in regions associated with up-regulated choline metabolism. Two different plans were calculated for these patients: one with 79 Gy delivered to the whole PTV, the other with, in addition, two simultaneous boost doses to the dominant intraprostatic lesion (corresponding to the BTV). These boost doses were equal to 100 and 105 Gy, delivered to the volumes defined by 60% and 70% of maximum prostatic uptake of the tracer, respectively. Poisson statistics based on the LQ model was adopted for TCP calculation, including also a sensitivity analysis for the α/β ratio (the parameter was varied between 1.5 and 10 Gy). NTCP was calculated for bladder, femoral head and rectum. The results show that a 10% increase in TCP is expected for the boost plans, without significant increase in NTCP compared to the literature IMRT data. Chang et al. also reported similar conclusions in a previous study investigating the potential of IMRT (28). Remarkably, the increase in TCP seems to be largely unaffected by the specific choice of α/β. This is encouraging given the current uncertainty in α/β.
Dose escalation for hypoxic tumours
Hypoxic tumours are also candidates for dose escalation due to increased radioresistance. Starting in 2007, Thorwarth et al. compared conventional IMRT plans with a uniform boost to a PET FDG-positive region (related to higher proliferation) and with DPBN, for 13 head and neck cancer patients (31). The DPBN consisted of a voxel-by-voxel dose prescription, based on the identification of hypoxic ‘spots’ in a PET map obtained by using as tracer fluoromisonidazole (FMISO), which is associated with hypoxia. In both cases the maximum dose per fraction was 2.2 Gy. For the DPBN approach, TCP calculations were employed in order to distribute the dose to single voxels to yield the same TCP in all voxels, taking into account hypoxia. The authors used their own TCP model, which assigned radiosensitivity to single voxels according to tracer retention. Results suggest that DPBN is a better strategy to increase TCP in hypoxic tumours; it allows localized dose escalation compared to the uniform boost. Uniform dose escalation translates into the over-treatment of some patients and under-treatment of others, and in general in higher doses to normal tissues. A similar comparison for eight head and neck cancer patients by Chang et al. yielded similar results (32). In addition to TCP, these authors also included NTCP evaluation in their analysis (parotid, mandible, spinal cord and brainstem). Even though similar TCP were obtained for uniform dose escalation and for voxel-by-voxel dose painting, due to the lower doses released to organs at risk (OARs) the latter yielded a higher uncomplicated TCP. FMISO was used also by others to demonstrate the feasibility of dose-escalation in hypoxic target volumes for head and neck cancer patients (33,34), with results consistent with those reported by Thorwarth et al. (31). Finally, it is worth mentioning that charged particles have also been discussed as a possible modality for the treatment of hypoxic tumours; Bassler and colleagues proposed an LET-painting approach for an oropharyngeal cancer case (35). The idea is to distribute the charged particles in order to concentrate the high-LET track sections (close to end of the range) on the hypoxic lesion, thus exploiting their higher relative biological effectiveness (RBE) compared to photons. Heavy charged particles like carbon and oxygen are the optimal candidates for this type of treatments, due to their high LET (and thus RBE) over the tumour volume (35). For a single patient they were able to demonstrate that a high-LET boost should increase the TCP. The size of the volume covered by the boost and the increase in TCP appeared to depend on the specific charged particles adopted, due to the different physics.
Local failures and dose painting
Recently Vogelius et al. proposed a failure-probability driven approach (36). The idea is that observed local-failure maps can be combined with the delineation of different target volumes based on both CT imaging and FDG uptake, in order to dose escalate and thus increase the TCP. Specifically, they defined five target volumes (CTV-FDG positive, GTV, CTV, CTV elective at low and high risk, in order of increasing size) for 20 HNSCC patients. These patients were extracted from a larger database, for which local failures and tumour recurrences were recorded. Thus, an observed probability of recurrence was assigned to each sub-volume. Dose distributions were calculated for each patient based on clinical prescription. TCP was calculated on a voxel basis for each of the five sub-volumes and then combined in an overall TCP. An expected TCP of about 70% was obtained, in line with observed clinical results. In a second step, the prescription was optimized maintaining the same integral dose to the target, but aiming at TCP maximization taking into account the observed failure probability. This resulted in increases in the expected TCP up to about 85%. These results are also supported by Grönlund and colleagues (37), who found a correlation between high FDG uptake regions and local recurrences. Based on that, they developed a dose-optimization strategy that increased the expected TCP by up to 14%, depending on the target characteristics for the specific patient.
NTCP: trends and issues
The use of NTCP models may help to create the plan that either minimizes radiation-induced side effects for a given pre-specified dose to the tumour, or that individualizes the tumour dose for a given acceptable NTCP, i.e., isotoxic prescribing (9,10,22,38).
Since the QUANTEC reviews (39) were published in 2010 there has been a progressive evolution in the philosophy of NTCP modelling, and most improvements in model predictive ability are based on the indications suggested by QUANTEC authors themselves.
Several studies (40-42) have demonstrated the importance of considering non-dosimetric factors such as health status, surgery, chemotherapy, base-line organ function or organ co-dependence (i.e., clinical variables). In addition, the prediction of individual risk of radiation-induced normal tissue complications can be further improved using biological and imaging predictors of radiation toxicity (43,44). Finally, organs are generally not homogeneous, so a procedure that reduces the organ dose distribution to a DVH or even to a single DVH parameter is unlikely to represent the fine-structure biology within the organ (45). Recently, models and methods that take into account the full three-dimensional (3D) dose distribution to evaluate dose-response relationship have been proposed (Figure 3) (46-49).
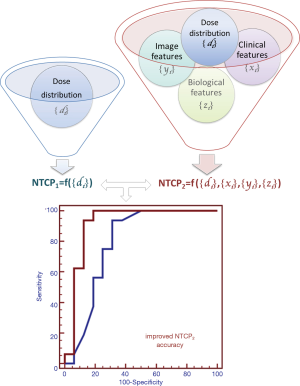
In the present review, we discuss some of these recent developments in the NTCP modelling.
Clinical features
The need to include non-dosimetric factors in NTCP models was one of the issues emphasized by QUANTEC. By using pseudo-clinical datasets with varying level of patient-specific factors, Onjukka et al. (40) compared the performance of different NTCP models subject to the influence of clinical confounding factors. The authors concluded that identifying confounding factors, and developing methods to quantify them, is more important than the choice of NTCP model itself. A method to generalize the LKB model in order to include clinical features (modified LKB) was first proposed in 2006 by Peeters et al. through a TD50 dose-modifying factor (50). This study included prostate cancer patients and the probability of developing late rectal toxicity was fitted with the original LKB and a modified model, in which a clinical feature (i.e., history of abdominal surgery) was taken into account by fitting subset-specific TD50 parameters. The ratio of these TD50s was the dose-modifying factor for that clinical feature. The modified LKB model resulted in a significantly better fit of the data and an improved estimation of NTCP compared with the original LKB model. In the same year, a different modelling approach—let’s call it phenomenological—was successfully proposed based on a multivariable logistic regression modelling framework (8) in which dose-volume metrics were combined with other patient- or disease-based prognostic factors using data-driven modelling to improve outcome prediction. The authors applied this approach to predict esophagitis and xerostomia after lung and head-and-neck radiation respectively, balancing complexity and model performance. Subsequently, a large number of published studies has explored the benefits of incorporating clinical factors into NTCP modelling using a logistic regression framework or advanced bioinformatics and computational statistics (51). Improved statistical machine learning methods such as the ‘least absolute shrinkage and selection operator’ (LASSO), artificial neural networks (ANN) and Bayesian model averaging (BMA), have been applied to address the multidimensional nature of NTCP predictions (52-56). Below, we focus our discussion on the results for two different organs at risk with different dose-volume effects: lungs and rectum.
Multiple clinical risk factors are known to influence the risk of developing radiation-induced lung disease. In recent years many efforts have focused on the determination of clinically useful predictors (dosimetric, physical and biological parameters) for acute or late pulmonary complications after thoracic RT; given the multivariate nature of lung complications, data-driven multivariate methods for NTCP modelling show much promise.
Age (57,58), the presence of cardiac comorbidities (59), and tumour location in the inferior part of the lung (60) are some of the clinical factors found to significantly increase the risk of lung toxicity. In particular, some clinical studies (56,61-63) have highlighted the role of heart irradiation in the development of radiation-induced lung toxicity. Of note, in contrast to those studies, Tucker et al. (64) found that the incorporation of heart parameters did not significantly improve radiation pneumonitis risk prediction.
As regards rectal NTCP modelling after RT for prostate cancer, the inclusion of factors such as drug prescription (anti-hypertensives and/or anti-coagulants), smoking history, previous abdominal surgery, pre-treatment morbidities (hypertension, cardiovascular history), diabetes mellitus and the presence of acute gastro-intestinal toxicity improved significantly the predictive power of different NTCP modelling approaches for rectal bleeding and high stool frequency or faecal incontinence (65-71).
Biological features
Biological markers of radiation-related side effects can be used to identify patients at increased risk of treatment-related injury. RT patients display different susceptibilities in their normal-tissue responses to identical radiation dose distributions; therefore, the inclusion of biological variability in NTCP modelling could improve predictive capability (43). According to Okunieff et al. (72) different categories of biomarkers for radiation effects on normal tissues can be defined: predictive (73), prognostic (74), diagnostic, and dosimetric (75). Predictive and prognostic factors, available respectively before or shortly after treatment, can be included in outcome models to aid prediction. Examples of the most investigated biological markers in RT outcome models are plasma biomarkers such as inflammatory cytokines and genetic variables such as single nucleotide polymorphism (SNPs).
Inflammatory cytokines, known factors in the pathogenesis of radiation pneumonitis, represent a possible serum biomarker for radiation-induced lung injury after thoracic irradiation (76). Transforming growth factor beta1 (TGF-β1) is the cytokine most extensively studied as a potential biomarker in this context. In the late 1990s and early 2000s elevation of plasma TGFβ1 above the baseline concentration at the end of RT was demonstrated to be a risk factor for symptomatic radiation-induced lung injury (77,78). In addition to TGF-β1, interleukin-6 (IL-6) and IL-8, IL-1β and tumour necrosis factor alpha (TNF-α), have been shown to be significantly correlated with the risk of lung toxicity (79,80) although conflicting results have been reported. However, only very few studies have proposed predictive models combining biological markers with lung dosimetric parameters. Analysing 78 lung cancer patients, the majority of whom received conventionally fractionated RT, Fu et al. (78) showed that, using the volume of lung irradiated to ≥30 Gy and TGF-β kinetics, patients could be divided into three groups having significantly different risks for developing symptomatic lung injury (6.9%, 22.8%, 42.9%, respectively, P=0.02). Stenmark et al. reported that combining IL-8, TGF-β1 and mean lung dose into a single model improved the predictive ability compared to either variable alone (model accuracy 80%, P<0.001) (81).
Additionally, RT-induced cardiac-cell damage and changes in the left ventricular loading conditions have been linked to several biomarkers including N-terminal pro-B-type natriuretic peptide (NT-proBNP) and troponins. In a retrospective study on left-sided breast cancer patients with or without chemotherapy, D’Errico et al. (82) analysed the relationship between NT-proBNP plasma levels and a large group of dosimetric parameters for the heart and left ventricle. Interestingly, in this study chemotherapy did not show a significant correlation with NT-proBNP plasma level elevations. Significant correlations between NT-proBNP plasma levels and some dosimetric parameters (high doses in small volumes) of the heart and left ventricle were found in those patients whose NT-proBNP values were above the pathological cut-off threshold (>125 pg/mL). More recently, in a prospective study on a relatively small number of breast cancer patients, another group (83) demonstrated that cardiac troponin T (cTnT) levels, detected by high-sensitive cardiac troponin T assay, increased during the entire duration of adjuvant breast RT. In addition, the increase in the cTnT release was positively associated with cardiac radiation doses and with minor changes in echocardiographic measurements revealing subclinical myocardial damage. There is a need to address with further studies the determination of biomarker plasma levels, as well as the optimal timing of biomarker measurements to predict the severity and progression of lung/cardiac damage after RT. A highly promising approach regarding biological features is radiogenomics (i.e., genomics of radiation toxicity). In recent years decisive evidence has been uncovered that genetic susceptibilities are linked to radiation-induced toxicity (84); this is the fruit of international collaboration, most notably based on the International Radiogenomics Consortium (RGC) (85) and the REQUITE project (86), and radiogenomic models including genetic, physical and clinical parameters are expected to reinforce personalized RT (86). The most frequently analysed radiogenomic biomarkers in an outcome-modelling framework are SNPs and genome-wide association studies (GWAS), with no a priori assumptions about which genes might be important, are ongoing in order to identify new genes associated with toxicity. Examples include the identification of SNPs related to radiation toxicity in prostate cancer treatment (85,87,88). Depending on the gene, copy number variations (CNVs) can span orders of magnitude more nucleotides than SNPs and are therefore thought to play an important role in the manifestation of toxicities. Coates et al. (89) explored the integration of both SNPs and CNVs with dosimetric and clinical variables in modelling radiation-induced rectal bleeding and erectile dysfunction in 62 prostate cancer patients who underwent curative hypofractionated RT and who were retrospectively genotyped for CNV and SNP rs5489 in the xrcc1 DNA repair gene. By analytical and data-driven approaches, the authors showed that biological variables added to the LKB model, and logistic-regression modelling improved classification performance over standard dosimetric models. Similarly, the study performed by Tucker et al. (90) on 141 non-small cell lung cancer patients receiving thoracic radiation therapy provides evidence that the SNPs associated with an increased risk of severe radiation pneumonitis were in genes for TGFβ, VEGF0, TNFα, XRCC1 and APEX1. The inclusion of those SNPs significantly improved the ability of the LKB model to predict the risk of severe radiation pneumonitis. However, from a statistical point of view, radiogenomic studies are challenging due to the high dimensionality of genomic data and thus great caution should be exercised when models based on multiple genetic-risk factors are both established and tested on the same patient cohort (91).
Image features
Anatomical, functional and molecular imaging for toxicity quantification is not new in RT; it has been on the increase in recent years (44). Image features are closely connected with anatomical, physiological, and molecular changes that characterize radiation effects on normal tissues and, similar to biomarker classification, they can be both predictive and prognostic. Recently, paediatric patients treated for ependymoma with proton therapy were analysed in relation to changes in normal-brain parenchyma on post-RT MR images (92). The authors implemented a voxel-by-voxel NTCP model for image changes showing dependence both on radiation dose and on linear energy transfer.
In addition, technological advances in biomedical imaging have resulted in the increasing popularity of quantitative clinical imaging studies (93). Regarding thoracic imaging, several groups have proposed CT-based methods to assess radiation-induced lung toxicity in terms of changes in density, quantitatively evaluated by Hounsfield unit changes. These studies have added information to standard clinical endpoints and enabled the identification of patient-specific susceptibility (94-97). In particular, Defraene and colleagues (97) explored the predictive value of CT imaging (obtained at baseline and 3 months after the end of treatment) for radiation pneumonitis in 130 lung cancer patients. Changes in Hounsfield units (ΔHU) were modelled as a function of the local dose using linear and sigmoidal fits. The authors suggest that a higher baseline lung density is significantly associated with a higher ΔHUmax. However, they noted that lung density changes did not necessarily imply symptomatic toxicity; radiation pneumonitis typically manifests itself as increases in density within the healthy lung tissue as observed in follow-up CT images.
Another example is the use of mid- or post-treatment FDG-PET to image and quantify the inflammatory processes as quantitative surrogates of radiation pneumonitis and acute esophagitis (98-100). Interestingly, in their study, Nijkamp et al. established a local-dose response model with post-RT FDG uptake response showing an improved prediction capability for severe acute esophagitis. Using instead pre-treatment non-target lung FDG-PET uptake (101), another group developed a predictive model for symptomatic radiation pneumonitis including both mean non-target lung uptake and mean lung dose, with a prediction accuracy of 80%.
In analogy with ‘genomics’, the increasing use of a wide range of specific imaging features (such as those inherent to texture analysis) is referred to as ‘radiomics’. Radiomic features and their mathematical definitions are independent of imaging modality and their definition can be found in the specialized literature (102). The potential of radiomics/texture techniques for the prediction of radiation-induced effects in organs at risk has been recently proposed (102-104). Texture analysis plays an important role in assessing the spatial organization of different tissues and organs, overcoming the limits of the classical global measures like mean CT number or standardised uptake value in PET images, which describe a region of interest as a homogeneous structure (102). Recently, texture analysis has been applied to radiation pneumonitis and parotid gland toxicity prediction. In a study on patients treated for oesophageal cancer (103), it has been demonstrated that quantitative measurement of dose-dependent texture changes between pre- and post-RT CT scans can differentiate between patients with and without clinical (grade >2) radiation pneumonitis. Twelve intensity- and texture-based classifiers demonstrated significantly increased changes for patients who developed radiation pneumonitis. The authors could not conclusively determine an optimal feature set and model due to the small number of cases (20 over 106) and they suggest that a non-linear classifier such as a neural network applied to a larger database might further improve classification accuracy. In their preliminary study on head and neck cancer patients, Scalco et al. (104) investigated the feasibility of using texture analysis on longitudinal CT images to characterize parotid variations induced by radiation. In particular, discriminant analysis based on volume and fractal dimension was able to predict the final parotid shrinkage with an accuracy of 71%. As with genomic studies, in order to build robust prediction models when using texture analysis, it is necessary to reduce the number of features by advanced machine learning techniques to avoid the risk of overfitting (105).
Beyond dose volume histogram and dose mapping
NTCP models for a given endpoint have traditionally relied on analyses that relate DVH characteristics to the risk of complications. However, DVH analyses disregard any spatial dose distribution information and possible inhomogeneity in regional organ radiosensitivity whereas it is known that functionality and repair processes are not uniformly distributed and are furthermore not independent of each other throughout organs or tissues. Therefore, the spatial information that DVH-based models are ‘blind’ to is likely to be important with respect to the development of toxicity (106).
In contrast to DVH-based NTCP models, Rutkowska et al. (47) proposed a radiobiologically based 3D model designed to include spatial effects and to explore the interface between theoretical radiobiology and clinical RT. The same group incorporated local lung-tissue damage and loss of global organ function into the modelling of radiation pneumonitis, consistent with the hypothesis of the alveolus as a functional subunit that can be regenerated from a single surviving stem cell (46).
Recently, 2D or 3D methods for dose distribution analysis (dose mapping), collectively referred to as pixel- or voxel-based methods, which evaluate local dose response patterns and go beyond the organ-based philosophy of NTCP modelling, have been proposed as alternative approaches to the DVH for predicting different toxicity endpoints after radiation therapy for prostate cancer (48,107-111) and for radiation-induced lung damage (49).
Image features predictive of radiation-induced effects on organs at risk could be successfully combined with dose distribution analysis to give information on dose effects at the spatial level and to elucidate radiobiological mechanisms.
Conclusions
The field of TCP and NTCP modelling is poised to go beyond the DVH-based models (2,4,5) though explicit recommendations on incorporating the extra patient-specific information discussed in this article, for specific tumours and OARs, are still lacking. The potential advantages that could arise from the ‘enhanced’ TCP and NTCP models are self evident, but there is a clear need for further research. This should involve both mathematical and technical aspects, as well as proof-of-concept pre-clinical and (ideally) clinical studies. Meanwhile the fact that current DVH- and population-based models are not perfect should not be used as a justification for not embracing ‘isotoxic’ tumour-dose prescribing. The ‘fixed tumour prescription for a fixed type of tumour’ paradigm is long past its ‘sell-by date’.
Acknowledgments
Funding: This work was partially supported by the National Institute for Nuclear Physics (INFN) CSN5 Call “MoVe IT”.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Durante, Giusi I. Forte, Giorgio Russo) for the series “Radiobiological models towards a personalized radiation oncology” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.06.03). The series “Radiobiological models towards a personalized radiation oncology” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys 1989;16:1623-30. [Crossref] [PubMed]
- Burman C, Kutcher GJ, Emami B, et al. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys 1991;21:123-35. [Crossref] [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [Crossref] [PubMed]
- Nahum A, Kutcher G. Biological Evaluation of Treatment Plans. In: Mayles P, Nahum A, Rosenwald JC. editors. Handbook of Radiotherapy Physics. Taylor & Francis, 2007:(731-71).
- Webb S, Nahum AE. A model for calculating tumour control probability in radiotherapy including the effects of inhomogeneous distributions of dose and clonogenic cell density. Phys Med Biol 1993;38:653. [Crossref] [PubMed]
- Nahum AE, Sanchez-Nieto B. Tumor control probability modeling: basic principles and applications in treatment planning. Phys Medica 2001;17:13-23.
- Källman P, Lind BK, Brahme A. An algorithm for maximizing the probability of complication-free tumour control in radiation therapy. Phys Med Biol 1992;37:871-90. [Crossref] [PubMed]
- El Naqa I, Bradley J, Blanco AI, et al. Multivariable modeling of radiotherapy outcomes, including dose-volume and clinical factors. Int J Radiat Oncol Biol Phys 2006;64:1275-86. [Crossref] [PubMed]
- Nahum AE, Uzan J. (Radio)biological optimization of external-beam radiotherapy. Comput Math Methods Med 2012;2012:329214 [Crossref] [PubMed]
- Uzan J, Nahum AE. Radiobiologically guided optimisation of the prescription dose and fractionation scheme in radiotherapy using BioSuite. Br J Radiol 2012;85:1279-86. [Crossref] [PubMed]
- Stavrev P, Stavreva N, Ruggieri R, et al. On differences in radiosensitivity estimation: TCP experiments versus survival curves. A theoretical study. Phys Med Biol 2015;60:N293-9.
- Chapman JD, Nahum AE. Radiotherapy Treatment Planning: Linear-Quadratic Radiobiology. Crc Press 2015;96:433-5.
- Parodi K. Vision 20/20: Positron emission tomography in radiation therapy planning, delivery, and monitoring. Med Phys 2015;42:7153-68. [Crossref] [PubMed]
- Ling CC, Humm J, Larson S, et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys 2000;47:551-60. [Crossref] [PubMed]
- Bentzen SM, Gregoire V. Molecular Imaging-Based Dose Painting: A Novel Paradigm for Radiation Therapy Prescription. Semin Radiat Oncol 2011;21:101-10. [Crossref] [PubMed]
- Franco P, Cante D, Sciacero P, et al. Tumor Bed Boost Integration during Whole Breast Radiotherapy: A Review of the Current Evidence. Breast Care (Basel) 2015;10:44-9. [Crossref] [PubMed]
- Arens AIJ, Troost EGC, Schinagl D, et al. FDG-PET/CT in radiation treatment planning of head and neck squamous cell carcinoma. Q J Nucl Med Mol Imaging 2011;55:521-8. [PubMed]
- Bentzen SM. Theragnostic imaging for radiation oncology: Dose-painting by numbers. Lancet Oncol 2005;6:112-7. [Crossref] [PubMed]
- Hall EJ. Dose-painting by numbers: A feasible approach? Lancet Oncol 2005;6:66. [Crossref] [PubMed]
- Nutting CM, Corbishley CM, Sanchez-Nieto B, et al. Potential improvements in the therapeutic ratio of prostate cancer irradiation: Dose escalation of pathologically identified tumour nodules using intensity modulated radiotherapy. Br J Radiol 2002;75:151-61. [Crossref] [PubMed]
- Sanchez-Nieto B, Nahum AE. The delta-TCP concept: a clinically useful measure of tumor control probability. Int J Radiat Oncol Biol Phys 1999;44:369-80. [Crossref] [PubMed]
- Uzan J, Nahum AE, Syndikus I. Prostate Dose-painting Radiotherapy and Radiobiological Guided Optimisation Enhances the Therapeutic Ratio. Clin Oncol (R Coll Radiol) 2016;28:165-70. [Crossref] [PubMed]
- Onjukka E, Uzan J, Baker C, et al. Twenty Fraction Prostate Radiotherapy with Intra-prostatic Boost: Results of a Pilot Study. Clin Oncol (R Coll Radiol) 2017;29:6-14. [Crossref] [PubMed]
- Ghobadi G, De Jong J, Hollmann BG, et al. Histopathology-derived modeling of prostate cancer tumor control probability: Implications for the dose to the tumor and the gland. Radiother Oncol 2016;119:97-103. [Crossref] [PubMed]
- van Lin ENJT, Fütterer JJ, Heijmink SWTPJ, et al. IMRT boost dose planning on dominant intraprostatic lesions: Gold marker-based three-dimensional fusion of CT with dynamic contrast-enhanced and 1H-spectroscopic MRI. Int J Radiat Oncol Biol Phys 2006;65:291-303. [Crossref] [PubMed]
- Casares-Magaz O, Van Der Heide UA, Rørvik J, et al. A tumour control probability model for radiotherapy of prostate cancer using magnetic resonance imaging-based apparent diffusion coefficient maps. Radiother Oncol 2016;119:111-6. [Crossref] [PubMed]
- Seppälä J, Seppänen M, Arponen E, et al. Carbon-11 acetate PET/CT based dose escalated IMRT in prostate cancer. Radiother Oncol 2009;93:234-40. [Crossref] [PubMed]
- Chang JH, Lim Joon D, Lee ST, et al. Intensity modulated radiation therapy dose painting for localized prostate cancer using 11C-choline positron emission tomography scans. Int J Radiat Oncol Biol Phys 2012;83:e691-6. [Crossref] [PubMed]
- Kuang Y, Wu L, Hirata E, et al. Volumetric modulated arc therapy planning for primary prostate cancer with selective intraprostatic boost determined by 18f-choline pet/ct. Int J Radiat Oncol Biol Phys 2015;91:1017-25. [Crossref] [PubMed]
- Lips IM, van der Heide UA, Haustermans K, et al. Single blind randomized Phase III trial to investigate the benefit of a focal lesion ablative microboost in prostate cancer (FLAME-trial): study protocol for a randomized controlled trial. Trials 2011;12:255. [Crossref] [PubMed]
- Thorwarth D, Eschmann SM, Paulsen F, et al. Hypoxia Dose Painting by Numbers: A Planning Study. Int J Radiat Oncol Biol Phys 2007;68:291-300. [Crossref] [PubMed]
- Chang JH, Wada M, Anderson NJ, et al. Hypoxia-targeted radiotherapy dose painting for head and neck cancer using (18)F-FMISO PET: a biological modeling study. Acta Oncol 2013;52:1723-9. [Crossref] [PubMed]
- Henriques de Figueiredo B, Zacharatou C, Galland-Girodet S, et al. Hypoxia imaging with [18F]-FMISO-PET for guided dose escalation with intensity-modulated radiotherapy in head-and-neck cancers. Strahlenther Onkol 2015;191:217-24. [Crossref] [PubMed]
- Hendrickson K, Phillips M, Smith W, et al. Hypoxia imaging with [F-18] FMISO-PET in head and neck cancer: Potential for guiding intensity modulated radiation therapy in overcoming hypoxia-induced treatment resistance. Radiother Oncol 2011;101:369-75. [Crossref] [PubMed]
- Bassler N, Toftegaard J, Lühr A, et al. LET-painting increases tumour control probability in hypoxic tumours. Acta Oncol 2014;53:25-32. [Crossref] [PubMed]
- Vogelius IR, Håkansson K, Due AK, et al. Failure-probability driven dose painting. Med Phys 2013;40:081717 [Crossref] [PubMed]
- Grönlund E, Johansson S, Montelius A, et al. Dose painting by numbers based on retrospectively determined recurrence probabilities. Radiother Oncol 2017;122:236-241. [Crossref] [PubMed]
- Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76:S10-9. [Crossref] [PubMed]
- Bentzen SM, Constine LS, Deasy JO, et al. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys 2010;76:S3-9. [Crossref] [PubMed]
- Onjukka E, Baker C, Nahum A. The performance of normal-tissue complication probability models in the presence of confounding factors. Med Phys 2015;42:2326-41. [Crossref] [PubMed]
- Cella L, Liuzzi R, Conson M, et al. Development of multivariate NTCP models for radiation-induced hypothyroidism: a comparative analysis. Radiat Oncol 2012;7:224. [Crossref] [PubMed]
- Cella L, Liuzzi R, Conson M, et al. Multivariate normal tissue complication probability modeling of heart valve dysfunction in hodgkin lymphoma survivors. Int J Radiat Oncol Biol Phys 2013;87:304-10. [Crossref] [PubMed]
- Bentzen SM, Parliament M, Deasy JO, et al. Biomarkers and surrogate endpoints for normal-tissue effects of radiation therapy: the importance of dose-volume effects. Int J Radiat Oncol Biol Phys 2010;76:S145-50. [Crossref] [PubMed]
- Jeraj R, Cao Y, Ten Haken RK, et al. Imaging for assessment of radiation-induced normal tissue effects. Int J Radiat Oncol Biol Phys 2010;76:S140-4. [Crossref] [PubMed]
- Trott KR, Doerr W, Facoetti A, et al. Biological mechanisms of normal tissue damage: Importance for the design of NTCP models. Radiother Oncol 2012;105:79-85. [Crossref] [PubMed]
- Rutkowska ES, Syndikus I, Baker CR, et al. Mechanistic modelling of radiotherapy-induced lung toxicity. Br J Radiol 2012;85:e1242-8. [Crossref] [PubMed]
- Rutkowska E, Baker C, Nahum A. Mechanistic simulation of normal-tissue damage in radiotherapy--implications for dose-volume analyses. Phys Med Biol 2010;55:2121-36. [Crossref] [PubMed]
- Acosta O, Drean G, Ospina JD, et al. Voxel-based population analysis for correlating local dose and rectal toxicity in prostate cancer radiotherapy. Phys Med Biol 2013;58:2581-95. [Crossref] [PubMed]
- Palma G, Monti S, D’Avino V, et al. A Voxel-Based Approach to Explore Local Dose Differences Associated With Radiation-Induced Lung Damage. Int J Radiat Oncol Biol Phys 2016;96:127-33. [Crossref] [PubMed]
- Peeters STH, Hoogeman MS, Heemsbergen WD, et al. Rectal bleeding, fecal incontinence, and high stool frequency after conformal radiotherapy for prostate cancer: normal tissue complication probability modeling. Int J Radiat Oncol Biol Phys 2006;66:11-9. [Crossref] [PubMed]
- El Naqa I, Li R, Murphy MJ. editors. Machine Learning in Radiation Oncology. Cham: Springer International Publishing, 2015.
- Xu CJ, van der Schaaf A, Van't Veld AA, et al. Statistical validation of normal tissue complication probability models. Int J Radiat Oncol Biol Phys 2012;84:e123-9. [Crossref] [PubMed]
- Lee TF, Chao PJ, Ting HM, et al. Using Multivariate Regression Model with Least Absolute Shrinkage and Selection Operator (LASSO) to Predict the Incidence of Xerostomia after Intensity-Modulated Radiotherapy for Head and Neck Cancer. PLoS One 2014;9:e89700 [Crossref] [PubMed]
- Cella L, Oh JH, Deasy JO, et al. Predicting radiation-induced valvular heart damage. Acta Oncol 2015;54:1796-804. [Crossref] [PubMed]
- Chen S, Zhou S, Yin FF, et al. Using patient data similarities to predict radiation pneumonitis via a self-organizing map. Phys Med Biol 2008;53:203-16. [Crossref] [PubMed]
- Lee S, Ybarra N, Jeyaseelan K, et al. Bayesian network ensemble as a multivariate strategy to predict radiation pneumonitis risk. Med Phys 2015;42:2421-30. [Crossref] [PubMed]
- Vogelius IR, Bentzen SM. A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol 2012;51:975-83. [Crossref] [PubMed]
- Dang J, Li G, Ma L, et al. Predictors of grade ≥ 2 and grade ≥ 3 radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with three-dimensional conformal radiotherapy. Acta Oncol 2013;52:1175-80. [Crossref] [PubMed]
- Nalbantov G, Kietselaer B, Vandecasteele K, et al. Cardiac comorbidity is an independent risk factor for radiation-induced lung toxicity in lung cancer patients. Radiother Oncol 2013;109:100-6. [Crossref] [PubMed]
- Hope AJ, Lindsay PE, El Naqa I, et al. Modeling radiation pneumonitis risk with clinical, dosimetric, and spatial parameters. Int J Radiat Oncol Biol Phys 2006;65:112-24. [Crossref] [PubMed]
- Huang EX, Hope AJ, Lindsay PE, et al. Heart irradiation as a risk factor for radiation pneumonitis. Acta Oncol 2011;50:51-60. [Crossref] [PubMed]
- Cella L, D’Avino V, Palma G, et al. Modeling the risk of radiation-induced lung fibrosis: Irradiated heart tissue is as important as irradiated lung. Radiother Oncol 2015;117:36-43. [Crossref] [PubMed]
- Valdes G, Solberg TD, Heskel M, et al. Using machine learning to predict radiation pneumonitis in patients with stage I non-small cell lung cancer treated with stereotactic body radiation therapy. Phys Med Biol 2016;61:6105-20. [Crossref] [PubMed]
- Tucker SL, Liao Z, Dinh J, et al. Is there an impact of heart exposure on the incidence of radiation pneumonitis? Analysis of data from a large clinical cohort. Acta Oncol 2014;53:590-6. [Crossref] [PubMed]
- Defraene G, Van den Bergh L, Al-Mamgani A, et al. The benefits of including clinical factors in rectal normal tissue complication probability modeling after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:1233-42. [Crossref] [PubMed]
- Rancati T, Fiorino C, Fellin G, et al. Inclusion of clinical risk factors into NTCP modelling of late rectal toxicity after high dose radiotherapy for prostate cancer. Radiother Oncol 2011;100:124-30. [Crossref] [PubMed]
- Hamstra DA, Stenmark MH, Ritter T, et al. Age and comorbid illness are associated with late rectal toxicity following dose-escalated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2013;85:1246-53. [Crossref] [PubMed]
- Cella L, D’Avino V, Liuzzi R, et al. Multivariate normal tissue complication probability modeling of gastrointestinal toxicity after external beam radiotherapy for localized prostate cancer. Radiat Oncol 2013;8:221. [Crossref] [PubMed]
- Ospina JD, Zhu J, Chira C, et al. Random forests to predict rectal toxicity following prostate cancer radiation therapy. Int J Radiat Oncol Biol Phys 2014;89:1024-31. [Crossref] [PubMed]
- D’Avino V, Palma G, Liuzzi R, et al. Prediction of gastrointestinal toxicity after external beam radiotherapy for localized prostate cancer. Radiat Oncol 2015;10:80. [Crossref] [PubMed]
- Tomatis S, Rancati T, Fiorino C, et al. Late rectal bleeding after 3D-CRT for prostate cancer: development of a neural-network-based predictive model. Phys Med Biol 2012;57:1399-412. [Crossref] [PubMed]
- Okunieff P, Chen Y, Maguire DJ, et al. Molecular markers of radiation-related normal tissue toxicity. Cancer Metastasis Rev 2008;27:363-74. [Crossref] [PubMed]
- Chen Y, Williams J, Ding I, et al. Radiation pneumonitis and early circulatory cytokine markers. Semin Radiat Oncol 2002;12:26-33. [Crossref] [PubMed]
- D’Alterio C, Avallone A, Tatangelo F, et al. A prognostic model comprising pT stage, N status, and the chemokine receptors CXCR4 and CXCR7 powerfully predicts outcome in neoadjuvant resistant rectal cancer patients. Int J Cancer 2014;135:379-90. [Crossref] [PubMed]
- d’Alesio V, Pacelli R, Durante M, et al. Lymph nodes in the irradiated field influence the yield of radiation-induced chromosomal aberrations in lymphocytes from breast cancer patients. Int J Radiat Oncol Biol Phys 2003;57:732-8. [Crossref] [PubMed]
- Fleckenstein K, Gauter-Fleckenstein B, Jackson IL, et al. Using biological markers to predict risk of radiation injury. Semin Radiat Oncol 2007;17:89-98. [Crossref] [PubMed]
- Anscher MS, Kong FM, Andrews K, et al. Plasma transforming growth factor beta1 as a predictor of radiation pneumonitis. Int J Radiat Oncol Biol Phys 1998;41:1029-35. [Crossref] [PubMed]
- Fu XL, Huang H, Bentel G, et al. Predicting the risk of symptomatic radiation-induced lung injury using both the physical and biologic parameters V(30) and transforming growth factor beta. Int J Radiat Oncol Biol Phys 2001;50:899-908. [Crossref] [PubMed]
- Oh JH, Craft JM, Townsend R, et al. A bioinformatics approach for biomarker identification in radiation-induced lung inflammation from limited proteomics data. J Proteome Res 2011;10:1406-15. [Crossref] [PubMed]
- Palmer JD, Zaorsky NG, Witek M, et al. Molecular markers to predict clinical outcome and radiation induced toxicity in lung cancer. J Thorac Dis 2014;6:387-98. [PubMed]
- Stenmark MH, Cai XW, Shedden K, et al. Combining physical and biologic parameters to predict radiation-induced lung toxicity in patients with non-small-cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys 2012;84:e217-22. [Crossref] [PubMed]
- D'Errico MP, Grimaldi L, Petruzzelli MF, et al. N-terminal pro-B-type natriuretic peptide plasma levels as a potential biomarker for cardiac damage after radiotherapy in patients with left-sided breast cancer. Int J Radiat Oncol Biol Phys 2012;82:e239-46. [Crossref] [PubMed]
- Skyttä T, Tuohinen S, Boman E, et al. Troponin T-release associates with cardiac radiation doses during adjuvant left-sided breast cancer radiotherapy. Radiat Oncol 2015;10:141. [Crossref] [PubMed]
- Barnett GC, Thompson D, Fachal L, et al. A genome wide association study (GWAS) providing evidence of an association between common genetic variants and late radiotherapy toxicity. Radiother Oncol 2014;111:178-85. [Crossref] [PubMed]
- Andreassen CN, Rosenstein BS, Kerns SL, et al. Individual patient data meta-analysis shows a significant association between the ATM rs1801516 SNP and toxicity after radiotherapy in 5456 breast and prostate cancer patients. Radiother Oncol 2016;121:431-9. [Crossref] [PubMed]
- De Ruysscher D, Defraene G, Ramaekers BLT, et al. Optimal design and patient selection for interventional trials using radiogenomic biomarkers: A REQUITE and Radiogenomics consortium statement. Radiother Oncol 2016;121:440-6. [Crossref] [PubMed]
- Kerns SL, Stock RG, Stone NN, et al. Genome-wide association study identifies a region on chromosome 11q14.3 associated with late rectal bleeding following radiation therapy for prostate cancer. Radiother Oncol 2013;107:372-6. [Crossref] [PubMed]
- Barnett GC, Kerns SL, Noble DJ, et al. Incorporating Genetic Biomarkers into Predictive Models of Normal Tissue Toxicity. Clin Oncol (R Coll Radiol) 2015;27:579-87. [Crossref] [PubMed]
- Coates J, Jeyaseelan AK, Ybarra N, et al. Contrasting analytical and data-driven frameworks for radiogenomic modeling of normal tissue toxicities in prostate cancer. Radiother Oncol 2015;115:107-13. [Crossref] [PubMed]
- Tucker SL, Li M, Xu T, et al. Incorporating single-nucleotide polymorphisms into the Lyman model to improve prediction of radiation pneumonitis. Int J Radiat Oncol Biol Phys 2013;85:251-7. [Crossref] [PubMed]
- Andreassen CN. A simulated SNP experiment indicates a high risk of over-fitting and false positive results when a predictive multiple SNP model is established and tested within the same dataset. Radiother Oncol 2015;114:310-3. [Crossref] [PubMed]
- Peeler CR, Mirkovic D, Titt U, et al. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiother Oncol 2016;121:395-401. [Crossref] [PubMed]
- Robbins ME, Brunso-Bechtold JK, Peiffer AM, et al. Imaging radiation-induced normal tissue injury. Radiat Res 2012;177:449-66. [Crossref] [PubMed]
- De Ruysscher D, Sharifi H, Defraene G, et al. Quantification of radiation-induced lung damage with CT scans: the possible benefit for radiogenomics. Acta Oncol 2013;52:1405-10. [Crossref] [PubMed]
- Bernchou U, Hansen O, Schytte T, et al. Prediction of lung density changes after radiotherapy by cone beam computed tomography response markers and pre-treatment factors for non-small cell lung cancer patients. Radiother Oncol 2015;117:17-22. [Crossref] [PubMed]
- Sharifi H, van Elmpt W, Oberije C, et al. Quantification of CT-assessed radiation-induced lung damage in lung cancer patients treated with or without chemotherapy and cetuximab. Acta Oncol 2016;55:156-62. [Crossref] [PubMed]
- Defraene G, van Elmpt W, Crijns W, et al. CT characteristics allow identification of patient-specific susceptibility for radiation-induced lung damage. Radiother Oncol 2015;117:29-35. [Crossref] [PubMed]
- McCurdy MR, Castillo R, Martinez J, et al. [18F]-FDG uptake dose-response correlates with radiation pneumonitis in lung cancer patients. Radiother Oncol 2012;104:52-7. [Crossref] [PubMed]
- Nijkamp J, Rossi M, Lebesque J, et al. Relating acute esophagitis to radiotherapy dose using FDG-PET in concurrent chemo-radiotherapy for locally advanced non-small cell lung cancer. Radiother Oncol 2013;106:118-23. [Crossref] [PubMed]
- Niedzielski JS, Yang J, Stingo F, et al. Objectively Quantifying Radiation Esophagitis with Novel Computed Tomography-Based Metrics. Int J Radiat Oncol Biol Phys 2016;94:385-93. [Crossref] [PubMed]
- Chaudhuri AA, Binkley MS, Rigdon J, et al. Pre-treatment non-target lung FDG-PET uptake predicts symptomatic radiation pneumonitis following Stereotactic Ablative Radiotherapy (SABR)Pre-treatment non-target lung FDG-PET uptake predicts radiation pneumonitis after SABR. Radiother Oncol 2016;119:454-60. [Crossref] [PubMed]
- Scalco E, Rizzo G. Texture analysis of medical images for radiotherapy applications. Br J Radiol 2017;90:20160642 [Crossref] [PubMed]
- Cunliffe A, Armato SG, Castillo R, et al. Lung texture in serial thoracic computed tomography scans: correlation of radiomics-based features with radiation therapy dose and radiation pneumonitis development. Int J Radiat Oncol Biol Phys 2015;91:1048-56. [Crossref] [PubMed]
- Scalco E, Fiorino C, Cattaneo GM, et al. Texture analysis for the assessment of structural changes in parotid glands induced by radiotherapy. Radiother Oncol 2013;109:384-7. [Crossref] [PubMed]
- Parmar C, Grossmann P, Bussink J, et al. Machine Learning methods for Quantitative Radiomic Biomarkers. Sci Rep 2015;5:13087. [Crossref] [PubMed]
- Deasy J, Saleh Z, Apte A. TU-C-BRA-01: Predicting Complication Probabilities Beyond the Dose-Volume Histogram: Dose-Mapping to Identify Critical Dose-Volume Risk Factors. Med Phys 2012;39:3901. [Crossref]
- Improta I, Palorini F, Cozzarini C, et al. Bladder spatial-dose descriptors correlate with acute urinary toxicity after radiation therapy for prostate cancer. Phys Med 2016;32:1681-9. [Crossref] [PubMed]
- Buettner F, Gulliford SL, Webb S, et al. Using dose-surface maps to predict radiation-induced rectal bleeding: a neural network approach. Phys Med Biol 2009;54:5139-53. [Crossref] [PubMed]
- Wortel RC, Witte MG, van der Heide UA, et al. Dose-surface maps identifying local dose-effects for acute gastrointestinal toxicity after radiotherapy for prostate cancer. Radiother Oncol 2015;117:515-20. [Crossref] [PubMed]
- Palorini F, Cozzarini C, Gianolini S, et al. First application of a pixel-wise analysis on bladder dose-surface maps in prostate cancer radiotherapy. Radiother Oncol 2016;119:123-8. [Crossref] [PubMed]
- Yahya N, Ebert MA, House MJ, et al. Modeling Urinary Dysfunction After External Beam Radiation Therapy of the Prostate Using Bladder Dose-Surface Maps: Evidence of Spatially Variable Response of the Bladder Surface. Int J Radiat Oncol Biol Phys 2017;97:420-6. [Crossref] [PubMed]


