
Surgical management of breast cancer in China: the Fudan University Shanghai Cancer Center experience
Introduction
Breast cancer is the most frequently diagnosed malignant tumor in Chinese women (15% of all cancer), and it ranks as the sixth leading cause of cancer-related death (1). Chinese breast cancer has had a significant upward trend in age-standardized incidence rates, especially early-stage (stage 0–II) cancer (1-3). Currently, surgery still dominates the treatment for early-stage breast cancer, although adjuvant therapy has rapidly developed (4).
A transition from “acceptable maximum treatment” to “minimally invasive procedures” in the concept of surgery has been continuing since Halsted described radical mastectomy (RM) in 1894 (5). On one hand, mastectomy has been replaced by breast conservative surgery (BCS). A series of large clinical trials and meta-analyses has demonstrated that the survival of patients who underwent mastectomy did not have an advantage, and these patients instead endured more harm than those who underwent BCS followed by radiation (6-9). The breast cancer incidence of Chinese women has undergone rapid growth in recent decades as has surgical management (2,10). However, the BCS rate in China is still lower than in developed countries, and modified radical mastectomy (MRM) is still the primary surgery (11,12). On the other hand, another minimally invasive model, sentinel lymph node biopsy (SLNB), which is recommended by American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) guidelines, offers better life quality for patients whose lymph nodes are negative and shows similar efficacy to axillary lymph node dissection (ALND) (4,13-15). Recent clinical trials, such as IBCSG 23-01 and Z0011, suggest that micro-metastasis or a limited number of positive sentinel lymph nodes (SLNs) should be considered to avoid ALND (16,17). This topic is still in discussion in China because of the limited pathological diagnosis methods. In addition, reconstruction surgery has undergone great advances in China, but the proportion of reconstruction surgery in total breast cancer surgery is still far behind international progress in this field.
Chinese oncologists have made substantial efforts to maintain a balance between patient safety, minimally invasive procedures and cost-benefit considerations, although there are some obstacles of accessibility to optimal treatment in China, including the developing socioeconomic status, low rate of early detection and lack of insurance coverage for many new medications. On the other hand, many breast cancer patients are diagnosed at a relatively late stage, making their chance of less-extensive surgery low. As we conduct the highest number of breast cancer surgeries in Shanghai and the 5-year survival rate has reached 93% for operable patients, we will introduce the surgical management mode of breast cancer in our cancer center, Fudan University Shanghai Cancer Center (FUSCC), and share our experiences of clinical exploration during the last decade in this review.
Pre-surgery treatment: cross-link of imaging and surgery
Imaging techniques have been widely used in the screening and diagnosis of breast cancer. In this section, we will introduce our routine work in pre-surgery diagnosis. In screening, we utilize mammography, ultrasonography and clinical examination in combination. Core-needle biopsy (CNB) is used in the preoperative diagnosis panel. Magnetic resonance (MR) guided biopsy is also discussed in our exploration.
Combined methods of screening are employed for screening in Chinese women. Early detection and diagnosis can reduce the breast cancer mortality, help maintain the shape of the breast and improve patient quality of life. In America, both the American Cancer Society (ACS) and U.S. Preventive Services Task Force (USPSTF) recommend screening for breast cancer from 40 years of age, while for women between 40 and 49 years of age, ACS advocates for annual screening testing (qualified recommendation) and USPSTF for biennial testing (C recommendation) (18,19). The benefits of mammography are not clear for females under 50 years of age. The resolution of mammography is not satisfactory for dense breasts, and ultrasonography can detect 27% or more malignant lesions within dense breasts as a supplementary tool (20). In China, the gland tissue of breast cancer patients tends to be denser, which is partially due to the ethnic characteristics of Asian females. Additionally, there is a higher proportion of young patients, whose breasts are prone to be denser, in China. Despite an increasing shift to older age, the mean age at the diagnosis of breast cancer in China is 45–55 years, which is much younger than in developed countries (21). To test the combined method in the Chinese community, FUSCC, along with community hospitals, practiced screening in the Qibao community, Shanghai. In Qibao, we screened a total of 13,183 females and diagnosed 33 cases of breast cancer. Up to 33.3% of those diagnosed with breast cancer underwent BCS, and 27.3% avoided undergoing ALND. Meanwhile, of the total patients at FUSCC in 2009, the BCS and axillary conserving rates were 14% and 8%, respectively. Based on the population-based study, the Chinese breast cancer guidelines [China Anti-Cancer Association guidelines for breast cancer diagnosis and treatment (CACA guidelines for short)] recommend annual or biennial mammography for all females over 40 years old, and ultrasonography is suggested for females with dense breasts (22). However, further research is needed to ascertain the benefits of the screening methods. In daily practice, the combined methods facilitate the early detection of breast cancer and improve the rate of less invasive surgery.
In the diagnosis procedure, CNB features accurate diagnosis, minimally invasive biopsy and high effectiveness, reducing the waiting time during surgery for pathology reports. In FUSCC, all biopsies were performed by open surgery before 2000. The rate of CNB in all biopsies was only 5% in 2002 and increased to 30–50% after 2010 (Figure 1) (Figure 1 based on data from FUSCC). Compared to open biopsy, CNB has the same accuracy and a lower coincidence rate (2–10% vs. <1%) (23).There are other less invasive methods in the diagnosis, such as fine-needle biopsy (FNB) and the Mammotome system. FNB shows relatively high undertriage and the possibility of false positive rates. The Mammotome system has a high accuracy rate, but it is limited due to relatively high cost and less available equipment. In all, CNB is recommended in pre-surgery diagnosis nationwide because of its accuracy and availability.
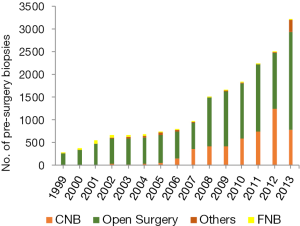
In addition to the widely used imaging methods mentioned above, we have explored MR guided biopsy in clinical practice. Imaging guided breast biopsy is divided into three categories: X-ray guided, ultrasound guided and MR guided biopsy. Ultrasound guided biopsy is usually the first choice because of its easy accessibility, and this technique is suggested for the underdeveloped area in China. X-ray guided biopsy has high diagnostic value along with low invasive lesions, and it is limited by the high equipment requirements. As a supplement to the above two methods, magnetic resonance imaging (MRI) is advantageous for its high sensitivity in detecting breast cancer (24), while its cost effectiveness requires further consideration. From 2011 to 2012, we performed MR guided biopsy in 38 cases and successfully observed five cases of invasive ductal carcinoma (IDC) and five of ductal carcinoma in situ (DCIS). This method can address many sub-clinical cases as well as improve the efficiency and targeting. MRI guided location evaluation and biopsy are suitable for “concealed” lesions that are unclear in mammography or ultrasonography but clear with MRI. Although MR guided biopsy has many advantages, difficulties remain, such as the disappearance of augmented loci during locating and strict requirements for the location system.
In all, the development of imaging allows for substantial work to be completed before surgery in non-invasive or minimally invasive procedures. These combined methods in screening make earlier diagnosis possible, and allow for less invasive surgery and better life quality. Additionally, CNB facilitates accurate diagnosis before surgery, with small lesions and a relatively low cost.
Surgical mode: breast conserving surgery and simple mastectomy (SM) are gradually increasing
The surgical modalities for breast cancer have undergone successive changes and revolutions with the development of adjuvant therapy and cancer biology. In this part, we will introduce the developing trend and current situation of our surgical mode, especially in terms of the increase in BCS and SM.
Since Halsted established RM in 1894, the surgery area has been undergoing a period of expansion and shrinkage. While Margottini and Urban favored removing the internal lymph node for extensive radical mastectomy (ERM), Patey and Auchincloss attempted MRM based on new anatomic knowledge about lymph vessels. Gradually, MRM began to dominate in the subsequent decades (5). In the 1970s, BCS was introduced into the breast cancer surgical field by Veronesi and Atkin, and it was successively supported by a series of prospective clinical trials and retrospective meta-analyses (6-9,24). It has been accepted by surgeons that BCS followed by radiation offers a somewhat similar survival benefit as mastectomy. Since then, BSC gradually advanced and became the first surgical choice for early breast cancer patients. For example, up to 60–70% of early-stage patients undergo BCS and 36% undergo mastectomy in the USA (11). Encouraged by BCS success, the concept of the surgical treatment mode evolved from the “acceptable maximum treatment” to “minimally invasive procedures.”
In China, the “minimally effective treatment” concept has been widely accepted and respected by doctors. BCS has been used since the mid-1990s, but its rate of use has been much slower in China than in developed countries (2). The specific and detailed operation guidelines of BCS were written in the CACA guidelines since the first edition. It contains detailed necessary requirements, indications, relative contraindications and absolute contraindications for BCS. After continuous revision, an expertise group from the CACA panel encouraged all early breast cancer patients who are willing to undergo a breast-conserving procedure, without contraindications, to choose BCS. The BCS rate has been increasing in recent decades, especially in 3-A-grade hospitals (10–30%) in China (12). However, limited by the relatively low diagnostic rate of early-stage breast cancer, shortage of radiotherapy equipment and conservative concepts among patients and doctors, the total BCS rate in China is approximately 10%, while mastectomy has a rate of 89% (25). Even in large modern cities, such as Beijing and Shanghai, the BCS rate varies at approximately 20% (12,25).
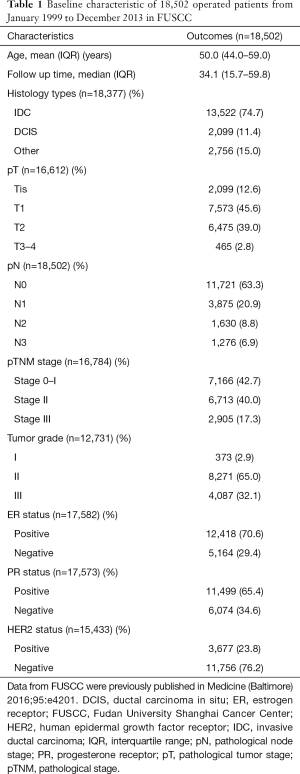
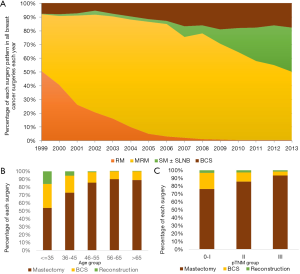
As one of the leading cancer centers in Shanghai, the surgery mode of FUSCC represents a relatively high level in China. In our breast cancer center, the patients are fighting cancer along with a whole multidisciplinary team (MDT), including surgeons, physicians, radiologists, radiotherapy doctors and pathologists. Equipped with standard diagnostic and radiotherapy equipment as well as thorough communications with doctors, increasing numbers of eligible patients are diagnosed earlier and are willing to undergo BCS as their first choice of treatment. Here we will retrospectively summarize the surgical trend in our center from 1999 to 2013. Table 1 summarizes the baseline characteristics of 18,502 patients who underwent breast cancer surgery from January 1999 to December 2013 in the FUSCC [Table 1 and Figure 2 based on data from Medicine (10)]. As the table shows, the median age of patients at the time of surgery was 50 years [interquartile range (IQR): 44.0–59.0], among which early breast cancer patients (stage 0–II) accounted for 82.7%. As shown in Figure 2A, the operation pattern in our FUSCC has continuously altered over the past 15 years [1999–2013]. In detail, MRM experienced an ascending trend before 2005 and gradually replaced ERM. Although it ranks the first among all prior types of surgeries, MRM use has been descending since 2005. In 2013, the MRM rate was lower than 50%. It should be noted that SM ± SLNB increased from 0.3% to 31.9%; meanwhile, BCS increased from 7.6% to 19.1% from 1999 to 2009. In recent years, the BCS rate remained approximately 18%, which was partially because MRI helps discover multi-focal or multi-center foci. Subgroup analysis revealed that patients under 35 years of age comprised the highest percentage group treated with BCS (29.9%, P<0.001) because young age was not a contraindication for BCS in the CACA guidelines (Figure 2B). Additionally, in pTNM 0–I stage patients, BCS accounted for 20.5% of cases (Figure 2C). The age and stage were two significant factors that influenced the surgery pattern in FUSCC. In summary, BCS and SM ± SLNB are gradually increasing, while RM and MRM are decreasing annually. The escalation of BCS and SM± SLNB represents advancement of the “minimally invasive” surgical concept in FUSCC. There are two main reasons that may explain this advancement. The first is the increasing proportion of early-stage breast cancer, which increases BCS eligible cases. The second is the promotion of SLNB in our center, which promotes SM ± SLNB surgery.
Full table
Based on the “minimally invasive procedures” concept, doctors have been focusing on key factors that influence the BCS for decades. There are two hot topics that we will discuss below. First is the negative margin topic. For surgeons, the negative margin is always the first aim. Discussions about the standard of the negative margin are frequently the topic of international conferences. In 2014, the American Society for Radiation Oncology (ASTRO) and Society of Surgical Oncology (SSO) (ASTRO/SSO) Consensus as well as 2015 St. Gallen guidelines issued criteria on the negative margin, which was set to “no ink on tumor or DCIS” (26,27). In China, there are two leading methods for pathological evaluations of BCS margins: radial sections perpendicular to the margin or shave sections of the margin. Regardless the chosen method, the 2015 edition of CACA guidelines suggest that pathologists color each surgical margin and define “no ink on tumor” as a “negative margin” as well. For those hospitals without standard pathologic equipment, “cavity shaving” is suggested as a supplementary method. It is supported by one blockbuster study from 2015, which was on cavity shaving margins (CSM). It demonstrated that CSM could decrease the positive margin rate from 34% to 19% and the second surgery rate from 21% to 10% (28). In FUSCC, we usually follow these criteria on margins, which can increase the BCS rate and decrease the second surgery rate.
Second concern is the approach for discovering local recurrence as soon as possible after BCS. To the best of our knowledge, true local recurrence usually occurs within 3–5 years, while the second primary tumor in the same breast usually grows after 10–15 years. Long-term follow-up studies showed that the local recurrence rate after BCS followed by radiotherapy varies between 3% to 22% (29). In FUSCC, the local recurrence rate of BCS followed by radiation was approximately 3% (30). We performed a retrospective analysis at our center in which recurrence free survival (RFS) and local recurrence free survival (LRFS) are two important endpoints. Until 2013, the 5-year RFS rate of BCS patients was 93.2% and 5-year LRFS rate was 96.5%, while for mastectomy, the rates were 87.6% and 96.0%, respectively, and they were probably influenced by stage (10). Multivariate analysis demonstrated that the lymph node status is a significant factor influencing the LRFS, especially in young patients (<50 years). The immunohistochemistry (IHC) subtypes is another factor. We found estrogen receptor positive (ER+) is a favorable characteristic for BCS patients. Additionally, when analyzing the annual recurrence pattern of mastectomy or lumpectomy, we observed a double-peak time distribution of the recurrence risk for mastectomy (a major peak at 2 years and moderate peak at 5 years), while there was only one peak at 5 years for BCS, which was confirmed by literature review (30). This finding suggests that the follow-up duration and schedule should be individually designed for BCS patients, which is also the trend for BCS management in China.
In summary, our experience, studies and guidelines facilitate our daily operation, promoting the implementation of BCS and SM ± SLNB. Investigators in FUSCC are making efforts to establish a recurrence prediction model for BCS patients, such as nomogram, to predict the recurrence possibility according to demographic and pathological characteristics. Additionally, our current goals are to customize tailored follow-up strategies, standardize salvage surgery for local recurrence patients and improve the BCS rate for patients after neoadjuvant chemotherapy (NAC).
SLNB: routinely conducted to save axilla
SLNB is another standard of care for clinical lymph node negative (cN0) patients, which involves interpreting the minimally invasive mode of local breast cancer treatment. Before 1993, ALND was the main operation for axillary staging until Krag et al. reported on SLNB in breast cancer treatment (31,32). The landmark Milan clinical trial, published in the NEJM in 2003, revealed that SLNB could accurately predict the axillary status, which laid the foundation for SLNB (13). Many prospective and retrospective analyses on SLNB supported that SLNB has similar efficacy to axillary dissection while offering a better quality of life for SLN negative patients (15,33). SLNB has been recommended in the ASCO guidelines and NCCN guidelines for 10 years, saving 60–75% patients from ALND and its associated side effects (14). In the 2015 edition, the CACA guidelines first suggested that SLNB should be performed as a routine procedure at the beginning of surgery as long as the hospitals have the relevant necessary equipment and techniques offered by MDT groups. Additionally, a qualified SLNB surgeon must achieve a greater than 90% success rate and less than 10% false negative rate in his or her personal SLNB experience. In China, combined methylene blue dye and radionuclide imaging are recommended, and a single marker could be used in well-practiced hospitals. Fluorescent dye has not yet been suggested as a routine method.
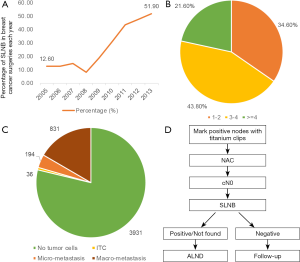
The CACA guidelines encourage surgeons to perform SLNB as a routine procedure. The actual percentage of patients in China who undergo ALND is up to 80%, including 60% with negative ALNs. The influences on the implementation of SLNB in China will be discussed below. First is the safety concern of the doctors. Hospitals in China with less advanced equipment are more willing to perform ALND for safety in the context of their limited techniques and cooperation with MDT team. Evaluation methods of SLN in China include touch imprint cytology (TIC) and intraoperative frozen section. In FUSCC, we use TIC as our routine method. The total accuracy rate of TIC in our center is 93.2%. In FUSCC experience, the SLNB rate is more than 50% among breast cancer surgeries, which is partially thanks to the MDT cooperation in our center. Figure 3A summarizes the SLNB trend of 4,992 SLNB cases in our center from 2005 to 2013 (Figure 3 based on data from FUSCC). The SLNB rate has a two-stage pattern; before 2008, SLNB remained stable at approximately 12%, and after 2008, SLNB rapidly increased up to 51.9% in 2013. This phenomenon could be explained by the reasons given by our MDT team and our prospective trials. Three hundred patients in our center with T1–2N0 tumors were randomly divided into the SLNB and ALND groups. The 5-year RFS results showed no significant difference between these two groups irrespective of subgrouping by pT1 and pT2. Figure 3B shows that the SLN number is grouped into three levels: 1–2, 3–4 and ≥4 nodes. Each level maintains a high percentage: 3–4 SLNs is most frequent one (43.8%) and ≥4 SLNs is the least frequent (21.6%). Figure 3C shows the final pathological diagnosis of SLNs. Up to 78.7% of reports lack a tumor in the lymph node, while the metastasis rate of SLN is 22.3% [16.6% for macro-metastasis, 3.9% for micro-metastasis and 0.7% for isolated tumor cells (ITC)]. In summary, MDT team work and relevant prospective studies help the implementation of SLNB in FUSCC. The second element that influences the widespread use of SLNB in China is salvage treatment for SLN positive patients. This topic remains under exploration and discussion. The IBCSG 23-01 trial divided SLN micro-metastasis patients into the ALND and follow-up groups (4,16). The 5-year follow-up results revealed that the local recurrence rate and overall survival had no difference between the two groups, while the ALND group had a higher chance of side effects, such as upper limb edema or movement disorder, suggesting that patients with micro-metastasis in the SLN should be relieved from ALND. Soon afterwards, the Z0011 and AMAROS (17,34) clinical trials explored the chance of exemption from ALND for SLN macro-meta stasis patients. The Z0011 trial showed no difference in recurrence events and the overall survival for patients with 1–2 SLN macro-metastasis who underwent BCS plus radiotherapy, while the AMAROS trial revealed that patients with a single SLN macro-metastasis could avoid ALND. Based on these major trials, the 2015 St. Gallen consensus supported promotion of the Z0011 experience in clinical practice. In China, the 2015 CACA guidelines showed that most CACA expertise supported the IBCSG 23-01 conclusion that micro-metastasis SLN patients with BCS followed by radiation may be spared from ALND, while ALND is still the standard of care for macro-metastasis. In FUSCC, we will consider not performing ALND for patients that undergo BCS followed by radiation to treat T1 stage and ER positive tumors. According to our summary, the ALND rate after SLNB in total SLNB cases is 26.8%, including 93.6% in macro-metastasis cases, 64.4% for micro-metastasis ones and 25% for ITC ones respectively. The survival analysis of the ALND and no-ALND groups showed that irrespective of the type of positive SLN, the 5-year-RFS is similar, with no significant difference, which encourages further use of SLNB in positive SLN cases. The third concern is the use of SLNB for NAC patients. Published studies showed that 30–40% of patients with positive lymph node became negative after NAC (35). Our data showed that ER-poor/HER-2 positive patients treated with trastuzumab achieved the highest negative conversion rate (79.6%) of axillary lymph nodes after NAC, suggesting that ER-poor and HER2-positive status may be a potential subtype of breast cancer that does not require ALND after NAC (36). The key question is how to accurately and safely evaluate these down-staged axillary lymph nodes. The Z1071 clinical trial demonstrated that the false negative rate (FNR) of SLNB on cN1 patients after NAC was up to 12.6%, while clipping positive nodes before NAC and removing more than 2 SLNs could diminish the FNR to 6.8% (37). However, the clipping technique is still debated in China because only several cancer centers perform SLNB after NAC. The 2015 CACA guidelines do not yet propose the routine use of SLNB for cN0 patients after NAC. In FUSCC, we designed our protocol to include SLNB after NAC. As shown in Figure 3D, adding titanium clips on positive lymph nodes with the help of ultrasound is the first step before NAC. After NAC, SLNB will be performed on cN0 patients. For those positive (or not found) results, ALND will be performed, while negative patients only require follow-up (Figure 3D). Whether this method works well requires long-term evaluation.
In summary, SLNB has been widely promoted in China, and it has been safely and effectively performed in our cancer center. It is critical to choose appropriate patients for SLNB to avoid unnecessary ALND and its side effects as well as to generate new and accurate SLNB methods for NAC patients. We hope for more long-term, prospective clinical trials to guide our procedures in clinical practice.
Reconstruction surgery: latissimus dorsi myocutaneous flap ± implantation is the most common surgery
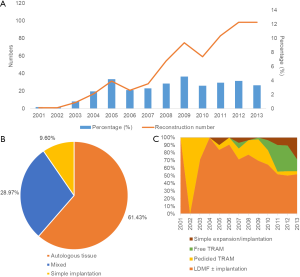
Reconstruction surgery is not a surgery to treat physiological disease; instead, it is a salvage surgery to address the psychological trauma and aesthetic defects. The first case of prosthesis implantation was reported in 1971. After 1970s, doctors started to combine local flaps and implantations together to improve the success rate. Since the 1980s, the emergence of an expander decreased surgery on the healthy breast. After 40 years of development, autologous tissue reconstruction has become the first choice for patients, including the transverse rectus abdominis musculocutaneous (TRAM) flap, free TRAM flap, latissimus dorsi myocutaneous flap (LDMF) and deep inferior epigastric artery perforator (DIEP) flap. Reconstruction surgeries could be divided into immediate breast reconstruction and delayed reconstruction according to the reconstruction time or be divided into autologous tissue reconstruction, implantation reconstruction and combined reconstruction according to the materials used for reshaping. In China, these reconstruction methods are performed, but the total rate of reconstruction surgery is only 4.5%, while it is up to 25.6% in developed countries. An investigation among 32 institutions from CACA showed that the main limitations for popularization among Chinese doctors include technical barriers to reconstruction, lack of team cooperation, a long period for training a qualified micro-surgeon and worries about local treatment safety (38). On the other hand, over-exaggerated fear of breast cancer from patients and poor economic foundation are the two main reasons patients do not choose reconstruction surgery. With the economic development in China, increasing numbers of patients are becoming concerned about their aesthetic needs. The 2015 CACA guideline introduced substantial information about reconstruction surgery to help promote it in China. Figure 4A displays the development curve of reconstruction surgery in our center from 2001 to 2013. The reconstruction cases increased with time, adding up to 573 immediate breast reconstruction cases. The percentage of reconstruction in total breast cancer surgery remained stable at approximately 4%. Figure 4B shows the composition of each reconstruction method. Autologous tissue reconstruction is the first choice (61.4%), while simple implantation is the last choice (9.6%), which is probably due to the expensive cost and complicated schedules. More specifically, LDMF with or without implantation is still the most common surgery at our center. Simple expansion/implantation surgery has rapidly increased in recent years. Pedicled-TRAM has been replaced by free-TRAM as the major abdominal flap reconstruction approach (Figure 4C) [Figure 4 based on data from Medicine (10)]. A retrospective study at our center analyzed 118 cases of reconstruction surgeries from 2006-2013 in FUSCC using f-TRAM techniques (39). The average surgical time is 7.72 h, and average hospitalization time after surgery is 10.73 days. In detail, the internal thoracic vessels are the first choice (72.0%). With respect to complications, only 3 cases experienced total flap necrosis. Survival analysis shows that the 5-year RFS for mastectomy is 88.3%, while the 5-year RFS of the reconstruction group is 92.3%, which is significantly higher than the mastectomy group and similar to the BCS group (92.3%). In summary, reconstruction surgery in our center is progressing at a steady pace and starting to lead on average in China, although it is far behind other International breast centers. We are making every effort in this area to make reconstruction a more viable option for more patients.
Conclusions
In conclusion, FUSCC achieves standard, distinctive surgical management experience based on guidelines, a series of studies and the context in China, which could be summarized in four parts. First, pre-surgery diagnosis in our center involves CNB guided by imaging to increase accuracy. Second, BCS and SM ± SLNB are increasing with time, which are characterized by individualized monitoring and evaluation strategies. Third, SLNB has been conducted as part of routine surgery. We developed our own protocol for SLNB after NAC with the aim of exploring how to better treat positive SLNs and down-staged SLNs after NAC. Finally, reconstruction surgery in our center is steadily progressing. Autologous tissue reconstruction, especially LDMF ± implantation, is the major approach, while implantation has remarkably increased. Our management experience is in line with international standards and considers patient survival and quality of life.
Acknowledgments
Funding: This work was supported by grants from the Research Project of Fudan University Shanghai Cancer Center YJ201401 (YZ Jiang); Training Plan of Excellent Talents in Fudan University Shanghai Cancer Center YJYQ201602 (YZ Jiang); National Natural Science Foundation of China 81572583 (ZM Shao), 81502278 (YZ Jiang), and 81372848 (ZM Shao); Research Fund for the Doctoral Program of Higher Education of China 20130071110057 (ZM Shao); Municipal Project for Developing Emerging and Frontier Technology in Shanghai Hospitals SHDC12010116 (ZM Shao); Cooperation Project of Conquering Major Diseases in Shanghai Municipality Health System 2013ZYJB0302 (ZM Shao); Innovation Team of Ministry of Education IRT1223 (ZM Shao); and Shanghai Key Laboratory of Breast Cancer 12DZ2260100 (ZM Shao).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.06.35). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Yu KD, Di GH, Wu J, et al. Development and trends of surgical modalities for breast cancer in China: a review of 16-year data. Ann Surg Oncol 2007;14:2502-9. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer Version 2.2016. Port Washington, PA: National Comprehensive Cancer Network; 2016.
- Plesca M, Bordea C, El Houcheimi B, et al. Evolution of radical mastectomy for breast cancer. J Med Life 2016;9:183-6. [PubMed]
- Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002;347:1227-32. [Crossref] [PubMed]
- . Early Breast Cancer Trialists' Collaborative G. Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N Engl J Med 1995;333:1444-55. [Crossref] [PubMed]
- Fisher B, Anderson S. Conservative surgery for the management of invasive and noninvasive carcinoma of the breast: NSABP trials. National Surgical Adjuvant Breast and Bowel Project. World J Surg 1994;18:63-9. [Crossref] [PubMed]
- van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol 2016;17:1158-70. [Crossref] [PubMed]
- Huang NS, Liu MY, Chen JJ, et al. Surgical management of breast cancer in China: A 15-year single-center retrospective study of 18,502 patients. Medicine (Baltimore) 2016;95:e4201 [Crossref] [PubMed]
- McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol 2009;16:2682-90. [Crossref] [PubMed]
- Zhang B, Song Q, Zhang B, et al. A 10-year (1999 ~ 2008) retrospective multi-center study of breast cancer surgical management in various geographic areas of China. Breast 2013;22:676-81. [Crossref] [PubMed]
- Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003;349:546-53. [Crossref] [PubMed]
- Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol 2005;23:7703-20. [Crossref] [PubMed]
- Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006;98:599-609. [Crossref] [PubMed]
- Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol 2013;14:297-305. [Crossref] [PubMed]
- Jagsi R, Chadha M, Moni J, et al. Radiation field design in the ACOSOG Z0011 (Alliance) Trial. J Clin Oncol 2014;32:3600-6. [Crossref] [PubMed]
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast Cancer Screening for Women at Average Risk 2015 Guideline Update From the American Cancer Society. JAMA 2015;314:1599-614. [Crossref] [PubMed]
- Siu ALU.S. Preventive Services Task Force. Screening for Breast Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164:279-96. [Crossref] [PubMed]
- Okello J, Kisembo H, Bugeza S, et al. Breast cancer detection using sonography in women with mammographically dense breasts. BMC Med Imaging 2014;14:41. [Crossref] [PubMed]
- Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. [Crossref] [PubMed]
- Committee of Chinese Breast Cancer Society. China Anti-Cancer Association guidelines for breast cancer diagnosis and treatment (2015 version). China Oncology 2015;25:692-754.
- Bruening W, Fontanarosa J, Tipton K, et al. Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann Intern Med 2010;152:238-46. [Crossref] [PubMed]
- Newman LA, Kuerer HM. Advances in breast conservation therapy. J Clin Oncol 2005;23:1685-97. [Crossref] [PubMed]
- Zhang BL, Sivasubramaniam PG, Zhang Q, et al. Trends in Radical Surgical Treatment Methods for Breast Malignancies in China: A Multicenter 10-Year Retrospective Study. Oncologist 2015;20:1036-43. [Crossref] [PubMed]
- Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533-46. [Crossref] [PubMed]
- Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Stages I and II Invasive Breast Cancer. Ann Surg Oncol 2014;21:704-16. [Crossref] [PubMed]
- Chagpar AB, Killelea BK, Tsangaris TN, et al. A Randomized, Controlled Trial of Cavity Shave Margins in Breast Cancer. N Engl J Med 2015;373:503-10. [Crossref] [PubMed]
- Huston TL, Simmons RM. Locally recurrent breast cancer after conservation therapy. Am J Surg 2005;189:229-35. [Crossref] [PubMed]
- Yu KD, Li S, Shao ZM. Different Annual Recurrence Pattern Between Lumpectomy and Mastectomy: Implication for Breast Cancer Surveillance After Breast-Conserving Surgery. Oncologist 2011;16:1101-10. [Crossref] [PubMed]
- Krag DN, Weaver DL, Alex JC, et al. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol 1993;2:335-9; discussion 40. [Crossref] [PubMed]
- Hsueh EC, Turner RR, Glass EC, et al. Sentinel node biopsy in breast cancer. J Am Coll Surg 1999;189:207-13. [Crossref] [PubMed]
- Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010;11:927-33. [Crossref] [PubMed]
- Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303-10. [Crossref] [PubMed]
- Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol 2005;23:9304-11. [Crossref] [PubMed]
- Li JW, Mo M, Yu KD, et al. ER-poor and HER2-positive: a potential subtype of breast cancer to avoid axillary dissection in node positive patients after neoadjuvant chemo-trastuzumab therapy. PLoS One 2014;9:e114646 [Crossref] [PubMed]
- Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and Resection of Clipped Node Decreases the False-negative Rate of Sentinel Lymph Node Surgery in Patients Presenting With Node-positive Breast Cancer (T0-T4, N1-N2) Who Receive Neoadjuvant Chemotherapy: Results From ACOSOG Z1071 (Alliance). Ann Surg 2016;263:802-7. [Crossref] [PubMed]
- Chen Y, Chen J, Chen J, et al. Current trends of breast reconstruction after mastectomy for breast cancer patients in China: a survey report. Zhonghua Zhong Liu Za Zhi 2014;36:851-7. [PubMed]
- Ying C, Jia-Ying C. Single-center report of 118 cases of free abdominal flaps for breast reconstruction. China Oncology 2013;8:576-83.


