
Unraveling bio-social hierarchy in cancer collective invasion: symbiosis between leaders and followers
Functional and phenotypic heterogeneity is a common feature of tumor cells. It can be caused by changes in the tumor microenvironment and genetic variations in the growing cell mass, while posing the problem in treating the tumor, considering various populations of heterogenic cells present. It has been also shown that cancer lesions may contain hierarchic populations of cells: some are tumorigenic and some are non-tumorigenic or even dormant (1). Immunohistochemistry and high-throughput single cell sequencing studies have reported that diverse cell subpopulations may contain different mutations, as well as protein expression profiles, resulting in varying degree of characteristics, including growth rates and responses to chemotherapeutics (2,3).
In 2005, National Cancer Institute (NCI) and National Human Genome Research Institute (NHGRI) have launched the Cancer Genome Atlas project which produced multi-dimensional maps of the key genomic alterations in more than thirty types of cancer (4). Although this groundbreaking project provided highly important new data about the genes that contribute to cancer development and progression, it did not consider intrinsic heterogeneous cell populations in each tumor mass as well as the microenvironmental diversity of the tumors (5). Some of the differences in physical behaviors, phenotypes and protein expression profiles of metastatic and non-metastatic tumor cells were revealed in a comprehensive study conducted by NCI/NIH network of Physical Sciences-Oncology Centers (PS-OC) (6).
The heterogenic plasticity of cancer cells is important in many hallmarks of cancer, including growth, progression, invasion and dissemination. As an example, in the process of epithelial-to-mesenchymal transition (EMT) leading to tumor invasion, the epithelial protein expression is inhibited in some subpopulations of tumor cells, while mesenchymal protein expression is elevated. This results in the loss of cell-cell adhesion and gain in the cell polarity, causing migration, enhanced dissemination from primary tumor and, ultimately, tumor metastasis (7). Additionally, non-EMT cancer cells and cells in the tumor microenvironment can cross-talk with EMT cancer cells in a paracrine manner (e.g., exosome release) setting the stage for metastatic spread of the primary tumor (8) and making intratumoral heterogeneity an important factor in tumor dissemination. While single-cell migration have been extensively studied, providing meaningful insights into the spread of blood-originated cancers and cells undergoing EMT, recent works show that besides the usually ascribed “solo” migrating individual cells, collective migration of a group of heterogeneous cancer cells increases their probability to disseminate to distant organs. A conventional clinically used assessment of tumor grades, for example, examines the morphology of the lesion and classifies invasiveness by identifying cell group(s) able to intrude into the normal tissue.
In collective cancer cell invasion, cohesive, multicellular structures further detach from the main tumor cell mass and disseminate using a blood or lymphatic vessel (9). In such a group, the cells maintain intercellular junctions and a “leader-follower cell” behavior may develop, as illustrated in Figure 1. In these cases, the leader cells, or phenotypically first cells in the group leading to tumor invasion, can generate traction forces by actomyosin-mediated protrusion and contractility, as well as ECM cleavage by MMP14 to promote the forward movement (10). Alternatively, they may lack actin protrusions extending into the ECM, which causes their position instability and generation of the forward push force by the cells behind the leading edge (11).
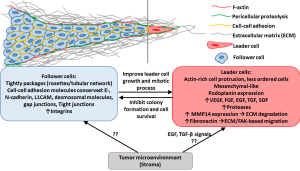
The question remains on how some of these cancer cells develop the EMT and migratory phenotype, and why the conversion is not uniform among the cells in the same lesion. Many efforts to characterize the molecular variations between tumors and even within the same tumor lesion have been pursued, but the topic remains a challenge to investigate. Organotypic models have been utilized to evaluate the multicellular invasion. Using 3D tumor cultures originated from primary breast cancers grown in K14-GFP-actin mice, Cheung et al have confirmed that in the process of collective invasion, leader cells expressed basal epithelial markers, such as keratin-K14 (12). Another study by Westcott et al. found that leader cells form epigenetically and morphologically distinct, duct-like spheroids, which are significantly more invasive as compared to the follower cells (13). The phenotype was maintained in the daughter cells after multigenerational sub-culturing of the leader and follower cells, showing a heritable feature. The above studies were conducted via expression analysis (post-sample fixation), thus, evaluation of the genome changes of the subpopulation in situ would allow greater understanding of the genetic profile that causes the development of the leader and follower cells.
Image-guided technology has been successfully employed for a few decades in oncology. It has been a gold standard in the modern healthcare for various therapies and surgical procedures, by assisting in term-volume assessment in diagnosis and therapy planning (14). Along with the improvements in the technology and resolution of the imaging probes with laser and high-resolution fluorescence-imaging strategies, the image-guided technology can nowadays enable a highly-specific tumor detection and image-guided surgery (15,16), as well as allow for image-guided biopsy for molecular and genomic profiling (17) and cancer therapy (18).
In the paper “Image-guided genomics of phenotypically heterogeneous populations reveals vascular signaling during symbiotic collective cancer invasion” published in May 2017 in Nat Communications, Konen et al. develop a novel in vitro technique, spatiotemporal genomic and cellular analysis (SaGa), which allows probing the heterogenic cancer populations in situ (19). The technique SaGa is utilized to specifically separate subpopulations of cells via cell transfection with a photoconvertible fluorophore of Dendra2, and the specific photoconversion in invading “leader” cancer cell. This method allows time-lapse live imaging, which tracks in vitro specific heterogenic sub-populations that take part in the process of invasion. The cells can later be separated via flow cytometry, thus enabling the genomic profiling of the subpopulation. SaGa further allowed tracking of expression via transcriptome profiling of the heterogeneous sub-populations taking part in collective tumor cell invasion. Clear differences were seen between the phenotypically distinguished “leaders” and “followers”. The authors reported that 788 candidate transcripts were upregulated in the leader cells as compared to the follower cells, and vice versa: 684 transcripts were upregulated in the follower cells as compared to the leader cells. Particularly vascular endothelial growth factor (VEGF) signaling transcript was increased in leader cells compared to follower cells, however, incubation with anti-VEGF antibody did not reduce the leader cells invasiveness, suggesting that the leader-follower cells utilize a non-canonical vascular signaling. Fibronectin-focal adhesion kinase (FAK) transcriptome data also revealed the increased phosphorylation of focal adhesion kinase at Y397 (pFAKY397) in leader cells, imparting them an ability to generate traction forces during migration. While there is no invasion possible without a leader cell on the edge of the tumor mass, the leader-follower relationship is a symbiotic one. The proliferation rate of the follower cells is higher than that of the leader cell, thus, the follower cells are necessary to produce the invading mass and to “push” the leader. Additionally, 70% of the leader cells were found to have mitotic defects as opposed to only 6% of the follower cells. Moreover, the secretome of the follower cells significantly improved the mitotic success of the leader cells. SaGa technology can be useful in isolating a phenotypically distinct sub-populations within a heterogeneous population of cells, such as in cancer lesions. Thus, it can in the future aid in isolating in situ cells based on their physical (e.g., migratory) or biological (e.g., proliferation rate, drug resistance) profiles, which can be phenotypically different when observed under the microscope. Further, SaGa can be utilized for evaluation of behavior of heterogeneous cell populations in the tumor and other tissues, as a function of internal and external stimuli which change phenotypic properties of the individual cells in the cell mass (20). Additional factors can be introduced to understand more about the role of microenvironmental effects on the change in cancer cell subtypes. As an example, the transition of tumor associated macrophages from anti-tumorigenic M1 to pro-tumorigenic M2 can affect the survival (21) and aggressiveness via paracrine loop with cancer cells (22,23).
Combining clinically and pre-clinically used image-guided technologies with SaGa may expand the platform from in vitro studies to in vivo approaches. As an example, image-guided micro-resection of tissue responding to the thermal stress after laser-induced injury enabled the analysis of genes involved. In this study Mackanos et al. used heat-shock-protein luciferase tagged transgenic mice to assess tissue heating profiles (24). SaGa is a novel important technique that can help to understand the drivers of tumor progression and develop novel anti-cancer therapeutic strategies, especially targeting the migratory subpopulations typically resistant to therapies. There are still many questions related to the hierarchical collective behaviors in the tumor mass. What drives the naissance of the cells to become “leaders”? How the cues in the tumor microenvironment will affect the relationship between cells in the various hierarchical categories? And other important questions still have to be elucidated. However, we can now witness that the sociologically observed phenomenon of “collective behavior” is true also on the micro-scale.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Lichao Sun (State Key Laboratory of Molecular Oncology, National Cancer Center (NCC)/Cancer Hospital, Chinese Academy of Medical Sciences (CAMS), Peking Union Medical College, Beijing, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.08.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baccelli I, Trumpp A. The evolving concept of cancer and metastasis stem cells. J Cell Biol 2012;198:281-93. [Crossref] [PubMed]
- McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 2015;27:15-26. [Crossref] [PubMed]
- Neelakantan D, Drasin DJ, Ford HL. Intratumoral heterogeneity: Clonal cooperation in epithelial-to-mesenchymal transition and metastasis. Cell Adh Migr 2015;9:265-76. [Crossref] [PubMed]
- Hampton T. Cancer genome atlas. JAMA 2006;296:1958.
- Aran D, Sirota M, Butte AJ. Systematic pan-cancer analysis of tumour purity. Nat Commun 2015;6:8971. [Crossref] [PubMed]
- Physical Sciences - Oncology Centers Network. A physical sciences network characterization of non-tumorigenic and metastatic cells. Sci Rep 2013;3:1449. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Neelakantan D, Zhou H, Oliphant MUJ, et al. EMT cells increase breast cancer metastasis via paracrine GLI activation in neighbouring tumour cells. Nat Commun 2017;8:15773. [Crossref] [PubMed]
- Friedl P, Locker J, Sahai E, et al. Classifying collective cancer cell invasion. Nat Cell Biol 2012;14:777-83. [Crossref] [PubMed]
- Wolf K, Wu YI, Liu Y, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol 2007;9:893-904. [Crossref] [PubMed]
- Ewald AJ, Brenot A, Duong M, et al. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell 2008;14:570-81. [Crossref] [PubMed]
- Cheung KJ, Gabrielson E, Werb Z, et al. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 2013;155:1639-51. [Crossref] [PubMed]
- Westcott JM, Prechtl AM, Maine EA, et al. An epigenetically distinct breast cancer cell subpopulation promotes collective invasion. J Clin Invest 2015;125:1927-43. [Crossref] [PubMed]
- Vannier MW, Marsh JL. Three-dimensional imaging, surgical planning, and image-guided therapy. Radiol Clin North Am 1996;34:545-63. [PubMed]
- Liu JT, Meza D, Sanai N. Trends in fluorescence image-guided surgery for gliomas. Neurosurgery 2014;75:61-71. [Crossref] [PubMed]
- Zhao T, Huang G, Li Y, et al. A transistor-like pH nanoprobe for tumour detection and image-guided surgery. Nat Biomed Eng 2016;1:0006.
- Marshall D, Laberge JM, Firetag B, et al. The changing face of percutaneous image-guided biopsy: molecular profiling and genomic analysis in current practice. J Vasc Interv Radiol 2013;24:1094-103. [Crossref] [PubMed]
- Lennon NJ, Adalsteinsson VA, Gabriel SB. Technological considerations for genome-guided diagnosis and management of cancer. Genome Med. 2016;8:112. [Crossref] [PubMed]
- Konen J, Summerbell E, Dwivedi B, et al. Image-guided genomics of phenotypically heterogeneous populations reveals vascular signalling during symbiotic collective cancer invasion. Nat Commun 2017;8:15078. [Crossref] [PubMed]
- Ware MJ, Tinger S, Colbert KL, et al. Radiofrequency treatment alters cancer cell phenotype. Sci Rep 2015;5:12083. [Crossref] [PubMed]
- Leonard F, Curtis LT, Ware MJ, et al. macrophage polarization contributes to the anti-tumoral efficacy of mesoporous nanovectors loaded with albumin-bound paclitaxel. Front Immunol 2017;8:693. [Crossref] [PubMed]
- Wyckoff J, Wang W, Lin EY, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 2004;64:7022-9. [Crossref] [PubMed]
- Qian B, Pollard JW. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010;141:39-51. [Crossref] [PubMed]
- Mackanos MA, Helms M, Kalish F, et al. Image-guided genomic analysis of tissue response to laser-induced thermal stress. J Biomed Opt 2011;16:058001 [Crossref] [PubMed]


