Intensity modulated radiotherapy for head-and-neck cancer: discussing safety of modern radiation techniques
Worldwide, head-and-neck cancer (HNC) accounts for more than 550,000 cases and 380,000 deaths annually. It is thereby the seventh most common cancer worldwide and also the seventh most common cause of death from cancer (1). Radiotherapy plays an important role in its treatment modalities. It can be recommended as definitive treatment with or without chemotherapy, adjuvant after surgery or in the treatment of local failure after surgery. Radiotherapy allows organ preservation and improved function preservation compared to surgery and can therefore be an elegant solution. The intensification of radiotherapy regimens and addition of concomitant chemotherapy has led to a decrease in local recurrences (LRs) and an improvement in overall survival, at the cost of increased toxicity (2,3). Despite these advances, however, loco-regional failure (LRF) remains an issue with up to 30% to 50% of patients relapsing loco-regionally within 5 years (4-6) .
The anatomy of the head and neck is complex because elements of the digestive-, respiratory-, nervous- and endocrine systems are located sometimes within millimetres of each other. Due to the complexity of this anatomical region, large and multiple target volumes and proximity to organs at risk (OARs), radiotherapy in this region is also complex. In addition, high dose radiotherapy (60–72 Gy) is necessary to cure patients. Irradiation of critical normal tissue can cause severe discomfort with increased acute and late morbidity (7). Newer techniques have been developed over the past two decades to improve delivery of radiotherapy with two aims. Firstly, to avoid critical normal tissue so as to decrease toxicity (8). Secondly, to administer a high enough dose to the tumour volume in order not to compromise control rates (9). To do so, techniques become more conformal and more precise by shaping the radiation beams more closely to the target volumes so that critical tissue is spared and toxicity is reduced (Figure 1).
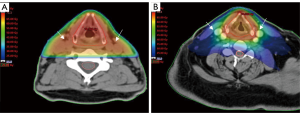
With technological advances in the medical field and the introduction of computed tomography (CT) scans in the late 1970s, also radiotherapy techniques were modernised. Using CT-scan information, the tumour could be visualised as well as OARs. Visualising organs such as parotid- and submandibular glands, oral mucosa, swallowing muscles and spinal cord allows them to be spared. Three-dimensional conformal radiotherapy (3DCRT) was implemented in the 1980s and remained the standard radiotherapy technique for the treatment of HNC in many hospitals until recently. With this technique the radiation beams are formed to fit the size and shape of the tumour better, allowing better sparing of OAR. Shortcomings of 3DCRT, however, are that it is administered in a robust fashion, usually with approximately three fields and a uniform dose in each field. This still causes a large volume of normal tissue receiving a high radiation dose.
To overcome these disadvantages, intensity modulated radiotherapy (IMRT) was implemented in the mid-1990s but only became more widespread in the last decade. Closer shaping to the tumour contour is made possible by aiming multiple photon beams from different directions and with adjusted intensities. This allows better sparing of the OARs resulting in less acute and late toxicity, especially xerostomia (9). Nutting et al. published the results of the PARSPORT trial (a phase 3 multicentre randomised controlled trial) in 2011. They concluded that sparing the parotid glands with IMRT significantly reduced the incidence of xerostomia and led to recovery of saliva secretion and improvements in associated quality of life (10). According to the NCCN guidelines, IMRT is now considered the standard of care for treating HNC (11).
A possible drawback of this more conformal technique that allows closer shaping to the tumour is the risk of missing areas that are at risk of harbouring tumour cells in proximity to the tumour. There are multiple reasons for a possible so-called geographical miss. A first reason might be the uncertainties in delineation of gross tumour volume (GTV) and clinical target volume (CTV). If an area is not defined as being at risk, it will consequently not be irradiated with this technique. This is in contrast to less conformal techniques where there is a spread of high dose around the target volume (Figure 1). Several delineation studies in HNC have been performed showing heterogeneity in delineation of GTV and CTV, resulting in radiotherapy inaccuracy, so this is a potential problem (12-16). A second reason might be inadequate immobilisation which allows patient movement, possibly resulting in an under dosage in target volumes and higher dose in OARs. Thirdly significant changes can occur in tumour volume during a course of radiotherapy due to shrinkage which can cause doses to be delivered incorrectly with possible under dosage in the target volume and a higher dose to OARs. 3DCRT is more forgiving to these changes or imperfections in target volume delineation than IMRT because the edges of the high dose are less sharp around the regions of interest.
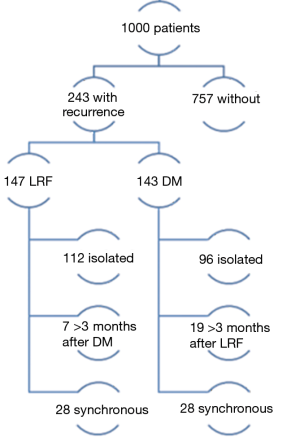
Several RCTs were already performed that proved that IMRT is safe and has a better toxicity profile than 3DCRT (9,10,17). Since IMRT is a more precise technique, it is interesting to learn where exactly recurrences occur, so where improvements can be realised. Recently published in the Journal of the American Medical Association Oncology, Leeman et al. presented their results concerning patterns of treatment failure after IMRT in locally advanced head-and-neck squamous cell carcinoma (HNSCC) (18). This article is especially interesting because they included 1,000 patients of which 147 had a loco-regional relapse (LRR). Many studies have been published regarding recurrence patterns but in these multicentre trials, the results may be affected by variations in the quality of IMRT of different centres and treatment centre volume causing true recurrence patterns to be unclear or biased by these artefacts. Therefore they retrospectively looked at 1,000 patients with stage III to IVB HNSCC treated at their centre alone. The median follow-up among surviving patients was 65.1 months. A total of 875 patients were treated with definitive IMRT with or without concurrent chemotherapy and 125 patients with oral cavity cancers (OCC) were treated with postoperative IMRT with or without concurrent chemotherapy. Regional nodal failures were delineated on diagnostic imaging and co-registered with the original treatment planning CT. The authors defined the recurrences according to the volume within the 95% isodose; in-field recurrences when 95% of the lesion was within the 95% isodose, marginal when 20–95% was within the 95% isodose and out- of-field when <20% was within the 95% isodose. This classification was used for GTV dose levels (70 Gy) as well as CTV dose levels (54–66 Gy). Dawson et al. and De Felice et al. published similar projects based on smaller patients’ numbers using the same cut-off values (19,20).
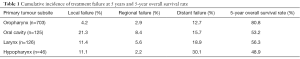
Of the 1,000 patients in the study of Leeman et al., 243 patients ultimately developed recurrences. One hundred and forty-seven relapsed loco-regionally of which 112 had an isolated loco-regional recurrence (LRR). Seven occurred more than 3 months after distant metastases (DM) and 28 were synchronous with DM. A total of 143 patients had DM, 96 of which were isolated, 19 occurred more than 3 months after LRF and 28 were synchronous (Figure 2). In Table 1, the cumulative incidences of recurrences at 5 years are shown. There is a noticeable difference between the groups of patients with OCC compared to the others. The former have more local and regional failure, despite the fact that 40.8% had tri-modality treatment. It would have been interesting to know how many of these recurrences in 5 years of follow-up were isolated local, regional or both and/or had DM, but this was not provided by the authors.
Full table
The authors did look at regional failure more closely. A total of 78 patients experienced regional nodal recurrence as the first site of relapse. It is not specified whether this was isolated or not. Sixty-three of these patients had non-OCC who did not undergo primary surgery. Of these, 55 (87.3%) relapsed in sites of treated gross disease. Only 8 relapsed in the elective neck alone (0.9%). For OCC, recurrence after surgery and adjuvant radiotherapy with or without chemotherapy occurred in the elective neck more often; 12 of 125 patients relapsed in the elective nodal CTV alone, mostly in the non-dissected neck. Three recurrences occurred out-of-field, in the anterior superficial lymphatic chain, which is not typically an area at risk. Although these recurrences occurred together with in-field recurrence, attention should be paid to this region according to the authors, for example by using a bolus.
Another aspect that would have been interesting to look at is the site of first relapse; local, regional, distal and whether it was isolated or synchronous. There were no marginal and no isolated out-of-radiation-field nodal recurrences in this treatment cohort of 1,000 patients.
Leeman et al. went further and investigated outcomes following LRF. In multivariate analyses, Karnofsky Performance score (KPS) greater than 70 and salvage surgery were associated with an improved survival after LRF. Patients with oropharyngeal cancer (OPC) that was human papilloma virus (HPV) or p16 positive had significant better overall survival than HPV negative OPC. Following DM, survival was associated with KPS greater than 70, 1 metastatic lesion, non-OCC, time to DM and palliative Cetuximab treatment. HPV or p16 status did not have an effect. Overall survival was better following isolated LRF than DM. Patients with a solitary metastasis radically treated with either surgery or radiotherapy, survived longer than patients who were not treated radically, although patients who did not receive local treatment had a lower KPS.
The strengths of this study are the large number of patients. It is a monocentric trial so the risk of having variations in IMRT quality is smaller than in multicentre trials. It was performed in Memorial Sloan Kettering Cancer Center which is a high volume centre. Consequently one can assume that the care offered to patients is optimal, based on clinical practice guidelines regarding radiotherapy dose, chemotherapy regimen and supportive care and the guidelines are used for delineation of target volumes. The combination of these factors allows true patterns of treatment failure to be visualised and their association with IMRT treatment fields or treatment-resistant disease, rather than recurrences due to sub-optimally delivered radiotherapy. It is also interesting how they looked at isolated regional recurrences (RRs) and isolated recurrences in the elective neck. Where exactly in the neck the recurrence occurs does not have a large impact on the possible treatment modalities for the recurrence, but it is does give us an idea of where we could improve radiotherapy in the future such as the possibility of dose escalation in the GTV or safety of dose de-escalation in the elective nodal CTV.
The strengths of this study however, are also partly their weakness. This study was done in a high volume centre where the radiotherapy techniques and delineations are presumably at their best. Unfortunately this might not be a realistic representation of the care provided to patients worldwide. As was shown by Wuthrick et al. (21), centres with smaller accrual rates to clinical trials have more recurrences and a lower overall survival and progression free survival than high accruing centres. Another limitation is that it is a retrospective cohort study and that patients were included from December 2001 to December 2013, which is an inclusion period of 12 years. Radiation dose may differ over time, as well as the technique applied. Delineation of elective nodal regions was adapted when evidence emerged showing low risk of recurrence in specific nodal regions in OPC patients. Regions that were omitted were level V in 2009, level IB in 2011 and higher retropharyngeal nodes in the uninvolved neck in 2013.
Leeman et al. were not the only ones to publish that relapse occurs predominantly in the GTV. Similar results were released by Chao et al. (22) and also by Nevens et al. (23). This in addition to more accurate imaging of primary tumour and pathological lymph nodes, more precise treatment planning and delivery, altered fractionation (2) and efficient concurrent chemotherapy (3) may offer the opportunity to lower the prescribed dose to the electively irradiated neck.
Nevens et al. investigated the safety of dose-reduction to the elective neck to 40 Gy in 233 patients (89.7% stage III and IV). Patients were included from 3 different randomised controlled trials and 71 patients developed a recurrence. Twenty had an isolated LR, 12 an isolated RR and 23 isolated DM. One out of 233 treated patients (0.4%) had an isolated recurrence in the elective nodal CTV receiving 40 Gy. In this study, 5 recurrences occurred outside the target volume, of which 2 in parotid glands that were not irradiated with IMRT and 3 after lymph node dissection. The same group also showed that dose de-escalation to the elective neck from 50 to 40 Gy resulted in significantly less dose to the pharyngeal constrictor muscles with less severe dysphagia at 3 months (24) and a trend towards less dysphagia at 6 months and less moderate late xerostomia without significant difference in LRF or OS (25). It has been shown that xerostomia and dysphagia have a significant impact on quality of life (26) but that compared to 3DCRT, IMRT does better (9,27).
In conclusion, IMRT allows sharper dose gradients around the target volumes. This allows better sparing of OARs, thereby causing less toxicity with a better quality of life after radiotherapy compared to 3DCRT. The fear of missing areas at risk of recurrence however is real. It is important to remember that as techniques become more accurate, other steps in the radiotherapy process become more important, such as delineation of the tumour, CTV delineation, CTV of elective nodes and OARs and patient immobilisation. In several studies IMRT has been shown to be safe. There are no more geographical misses compared to 3DCRT, similar control rates and overall survival. We have to remember, however, that this technique substantially increases the complexity and importance of correct contouring to reduce LRR and toxicity even more. On the other hand, there is a mountain of evidence showing that recurrences most often occur in GTV and regions that originally received a high dose (18,19,22,28). Now that it has once again been shown that recurrences occur in regions in or close to GTVs, research should be done to investigate if delineations are done correctly and if diagnostic imaging is interpreted correctly, possibly comparing it to the golden standard; pathology specimens. If correct delineation is confirmed and radio-resistant tumour sub-volumes have been identified, the benefit of dose-escalation can be investigated. Recurrences in elective lymph node regions alone, without recurrences in the primary tumour region or pathological lymph nodes, are very rare (<1%) in the studies mentioned above. This could be an argument for reducing the dose to the elective nodal regions. Studies using 40 Gy have proven successful, causing less toxicity and having similar outcomes as 50 Gy. Further research is needed to validate this.
Even though the studies mentioned above showed that recurrences occur in regions that received a high radiation dose, we have to also keep in mind that these studies were carried out in high volume centres. It has been shown that there is a significant difference in oncological outcome between high accruing and low accruing centres. If accrual rates in clinical trials are a representable substitute for the number of patients a centre treats, this means that patients treated in low volume centres may have a worse oncological outcome. If recurrence patterns were investigated in low volume centres, we may find different results than the ones mentioned above. We may for example find more relapses outside elective nodal CTV if delineation is not done correctly or in elective nodal CTV if dose is not delivered correctly. We want to therefore also emphasise the importance of training radiation oncologists in delineation of GTV’s and CTV’s, formulating clear protocols and creating clear delineation guidelines that are understandable and applicable for everyone, especially in the IMRT era where an accurate target volume delineation is of the utmost importance.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor San-Gang Wu (Department of Radiation Oncology, Xiamen Cancer Center, The First Affiliated Hospital of Xiamen University, Xiamen, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.07.16). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 2006;368:843-54. [Crossref] [PubMed]
- Pignon JP, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4-14. [Crossref] [PubMed]
- Due AK, Vogelius IR, Aznar MC, et al. Recurrences after intensity modulated radiotherapy for head and neck squamous cell carcinoma more likely to originate from regions with high baseline [18F]-FDG uptake. Radiother Oncol 2014;111:360-5. [Crossref] [PubMed]
- Bayman E, Prestwich RJ, Speight R, et al. Patterns of failure after intensity-modulated radiotherapy in head and neck squamous cell carcinoma using compartmental clinical target volume delineation. Clin Oncol (R Coll Radiol) 2014;26:636-42. [Crossref] [PubMed]
- Jeong S, Yoo EJ, Kim JY, et al. Re-irradiation of unresectable recurrent head and neck cancer: using Helical Tomotherapy as image-guided intensity-modulated radiotherapy. Radiat Oncol J 2013;31:206-15. [Crossref] [PubMed]
- Marta GN, Silva V, de Andrade Carvalho H, et al. Intensity-modulated radiation therapy for head and neck cancer: systematic review and meta-analysis. Radiother Oncol 2014;110:9-15. [Crossref] [PubMed]
- Jellema AP, Slotman BJ, Doornaert P, et al. Impact of radiation-induced xerostomia on quality of life after primary radiotherapy among patients with head and neck cancer. Int J Radiat Oncol Biol Phys 2007;69:751-60. [Crossref] [PubMed]
- Ghosh-Laskar S, Yathiraj PH, Dutta D, et al. Prospective randomized controlled trial to compare 3-dimensional conformal radiotherapy to intensity-modulated radiotherapy in head and neck squamous cell carcinoma: Long-term results. Head Neck 2016;38:E1481-7. [Crossref] [PubMed]
- Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011;12:127-36. [Crossref] [PubMed]
- NCCN. Head and Neck Cancers (Version 2.2017). Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- Njeh CF. Tumor delineation: The weakest link in the search for accuracy in radiotherapy. J Med Phys 2008;33:136-40. [Crossref] [PubMed]
- Jeanneret-Sozzi W, Moeckli R, Valley JF, et al. The reasons for discrepancies in target volume delineation: a SASRO study on head-and-neck and prostate cancers. Strahlenther Onkol 2006;182:450-7. [Crossref] [PubMed]
- O'Daniel JC, Rosenthal DI, Garden AS, et al. The effect of dental artifacts, contrast media, and experience on interobserver contouring variations in head and neck anatomy. Am J Clin Oncol 2007;30:191-8. [Crossref] [PubMed]
- Riegel AC, Berson AM, Destian S, et al. Variability of gross tumor volume delineation in head-and-neck cancer using CT and PET/CT fusion. Int J Radiat Oncol Biol Phys 2006;65:726-32. [Crossref] [PubMed]
- Hong TS, Tomé WA, Harari PM. Heterogeneity in head and neck IMRT target design and clinical practice. Radiother Oncol 2012;103:92-8. [Crossref] [PubMed]
- Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys 2006;66:981-91. [Crossref] [PubMed]
- Leeman JE, Li JG, Pei X, et al. Patterns of Treatment Failure and Postrecurrence Outcomes Among Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma After Chemoradiotherapy Using Modern Radiation Techniques. JAMA Oncol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Dawson LA, Anzai Y, Marsh L, et al. Patterns of local-regional recurrence following parotid-sparing conformal and segmental intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2000;46:1117-26. [Crossref] [PubMed]
- De Felice F, Thomas C, Barrington S, et al. Analysis of loco-regional failures in head and neck cancer after radical radiation therapy. Oral Oncol 2015;51:1051-5. [Crossref] [PubMed]
- Wuthrick EJ, Zhang Q, Machtay M, et al. Institutional clinical trial accrual volume and survival of patients with head and neck cancer. J Clin Oncol 2015;33:156-64. [Crossref] [PubMed]
- Chao KS, Ozyigit G, Tran BN, et al. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2003;55:312-21. [Crossref] [PubMed]
- Nevens D, Duprez F, Daisne JF, et al. Recurrence patterns after a decreased dose of 40Gy to the elective treated neck in head and neck cancer. Radiother Oncol 2017;123:419-23. [Crossref] [PubMed]
- Nuyts S, Lambrecht M, Duprez F, et al. Reduction of the dose to the elective neck in head and neck squamous cell carcinoma, a randomized clinical trial using intensity modulated radiotherapy (IMRT). Dosimetrical analysis and effect on acute toxicity. Radiother Oncol 2013;109:323-9. [Crossref] [PubMed]
- Nevens D, Duprez F, Daisne JF, et al. Reduction of the dose of radiotherapy to the elective neck in head and neck squamous cell carcinoma; a randomized clinical trial. Effect on late toxicity and tumor control. Radiother Oncol 2017;122:171-177. [Crossref] [PubMed]
- Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol 2008;26:3770-6. [Crossref] [PubMed]
- Rathod S, Gupta T, Ghosh-Laskar S, et al. Quality-of-life (QOL) outcomes in patients with head and neck squamous cell carcinoma (HNSCC) treated with intensity-modulated radiation therapy (IMRT) compared to three-dimensional conformal radiotherapy (3D-CRT): evidence from a prospective randomized study. Oral Oncol 2013;49:634-42. [Crossref] [PubMed]
- Johansen S, Norman MH, Dale E, et al. Patterns of local-regional recurrence after conformal and intensity-modulated radiotherapy for head and neck cancer. Radiat Oncol 2017;12:87. [Crossref] [PubMed]