
Tumor metabolism and prognostic role of EZH2 in non-small cell lung cancer
EZH2 in non-small cell lung cancer (NSCLC)
Epigenetic parameters—as DNA methylation and histone acetylation - play pivotal roles in carcinogenesis (1). Polycomb group (PcG) proteins are epigenetic effectors that maintain the silenced state of genes. The enhancer of zeste homolog 2 (EZH2) is one of the most important components of the polycomb repressive complex 2 (PRC2) and plays an important role in tumorigenesis and cancer progression (2). EZH2 has also been shown to be a key regulator of tumor angiogenesis (3). Overexpression of EZH2 has been associated to patients’ prognosis in various malignant tumors; on the other hand, recent studies have hypothesized that EZH2 is involved in drug resistance in ovarian cancers and its overexpression is detected in cisplatin-resistant lung cancer cells. Nevertheless, the relationship between the EZH2 expression and the development of chemotherapy resistance to NSCLC is still unclear, as well as its prognostic role (4-7).
In a retrospective study including 195 patients affected by NSCLC, EZH2 expression has been demonstrated to be negative in normal lung tissues but positive in lung cancer tissues with heterogeneous levels of expression. In the same study, the authors showed that overexpressing EZH2 increased VEGF-A expression, promoted cell proliferation and cell cycle progression, migration and invasion of lung cancer cells, while silencing EZH2 had the opposite effects, thus suggesting that EZH2 promotes lung cancer progression and correlates with increased tumor size, high VEGF-A expression and AKT activation (8). Interestingly, the activation of the VEGF/VEGFR-2 pathway in malignant cells overexpressing VEGFR-2 promotes cell migration, proliferation, and survival by upregulating EZH2 expression, thus favoring platinum-resistance and reducing sensitivity to VEGFR-2 targeted therapy (9). A recent meta-analysis confirmed that EZH2 overexpression is associated with poor prognosis in terms of overall survival (OS) in patients affected by NSCLC, and in particular in Asian population, in lung adenocarcinomas and in stage I disease (3,10). In addition, Behrens et al. showed that higher EZH2 expression in adenocarcinoma seems associated with both worse recurrence-free survival (RFS) and OS in patients with stages I–III surgically resected lung adenocarcinomas (11). A recent retrospective study showed a poorer response to cisplatin-based chemotherapy in EZH2-positive than in EZH2-negative patients affected by stage IIIB and IV NSCLC; positive EZH2 status resulted associated with a poorer prognosis, statistically significant in adenocarcinoma histology, but not in squamous cell carcinoma (12). Another retrospective study evidenced that when EZH2 is suppressed, p53 upregulated modulator of apoptosis (PUMA) expression is concomitantly induced, thus resulting in an elevated cisplatin-induced apoptosis; as a consequence, in EZH2-positive NSCLC cells, PUMA is suppressed and apoptosis inhibited (13). In conclusion, the hyper-expression of EZH2 seems associated to poorer prognosis, either promoting lung cancer progression through cell cycle regulation or inhibiting cisplatin-induced apoptosis or reducing tumor sensitivity to target therapies.
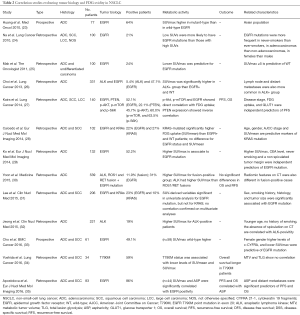
As the hyper-expression of EZH2 seems related to acquired platinum-resistance, it could be interesting to classify patients basing on this biological feature in order to choose the most appropriate treatment. Anyhow, in most of studies evaluating the role of EZH2 in NSCLC, several clinic-pathological factors have been associated to EZH2 hyper-expression (Table 1). In addition, EZH2 expression has been determined by immunohistochemistry (IHC), using several antibodies at different dilutions; other authors preferred the real-time PCR system. In those using IHC, the immune-reactivity levels of EZH2 were estimated basing both on the intensity of expression (0, negative; 1, weak; 2, moderate; and 3, intensive) and the proportion of positive cells (with different cut-off values) (Table 1). Thus, there is still not any agreement regarding the method and cut-off values for evaluating the presence of hyper-expression of EZH2 on cancer tissue samples. Nevertheless, the metabolic activity of cancer measured by 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) seems associated with the biological features of cancer cells such as proliferation and the histological type; moreover, there seems to be a direct association between the expression of EZH2 and the uptake of FDG measured by the maximum standardized uptake value (SUVmax) in NSCLC (14). In the following session we will illustrate these aspects by providing results of the available data concerning NSCLC metabolism and its correlation with molecular or genetic features.

Full table
Metabolic activity and correlation with NSCLC biology
Recently, Toyokawa et al. published for the first time in NSCLC patients a direct association between metabolic activity on 18F-FDG PET (SUVmax) and tumor expression of EZH2 (14). The authors conducted a retrospective analysis on EZH2 protein expression in 268 patients with resected NSCLC, all investigated with 18F-FDG PET prior to surgery. The results of their study documented that the SUVmax of EZH2-positive lesions was significantly higher than in EZH2-negative cases; these findings were proven true for the entire cohort of NSCLC patients and for the adenocarcinoma (ADC) group analyzed separately. Interestingly, the majority of patients with squamous cell carcinoma (SCC), corresponding to 88.9% of the cases, were EZH2-positive. This high prevalence was in addition associated with a no statistically significant difference in terms of SUVmax based on EZH2 expression for SCC. The abovementioned results appear in line with what reported in literature (10-12); indeed, the presence of EZH2 hyper-expression in NSCLC is associated with a more aggressive tumor behaviour, and consequently a significantly poorer DFS and OS in comparison to EZH2-negative patients. Comprehensively, the multivariate analysis in the study form Toyokawa et al. (14) revealed as independent predictors of EZH2-positivity in NSCLC lesions a higher SUVmax, the presence of vascular invasion and a SCC histology. The particular value of this study, despite its retrospective nature, resides in its capability to document for the first time a direct association between tumor metabolism on 18F-FDG PET and hyper-expression of EZH2 in NSCLC. Moreover, the report confirms indirectly the expected prognostic role of above mentioned factors.
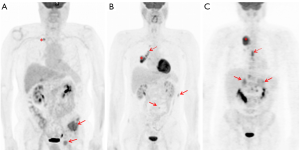
The prognostic significance of SUVmax in NSCLC, however, is not a new discovery. Several meta-analyses, of which the last one performed on 36 studies and comprising an overall cohort of 5,807 patients (15), confirm that a high metabolic activity, defined by either SUVmax, metabolic tumor volume (MTC) or total lesion glycolysis (TLG), predicts a higher risk of recurrence or death in patients with NSCLC. The rationale behind these findings relies on the high glucose dependence of rapidly growing tumor cells that change their metabolic profile to a much lower rate of oxidative phosphorylation and a high rate of glycolysis followed by lactic acid production, even under aerobic conditions (16). This phenomenon is called the “Warburg effect” (17) and allows for the metabolic characterization of malignant tissues by means of the radiolabeled fluorinated glucose analogue 18F-FDG (fluorine-18-2-fluoro-2-deoxy-D-glucose). The enhanced metabolic activity in malignant tumor cells, and specifically in NSCLC lesions, has been associated to many oncogenic signaling pathways (18-22) (Table 2). Some examples are represented by the PI3K-AKT or RAS-MAPK pathways, PTEN, p-AKT, p-mTOR and p-S6K, ALK-rearrangement, ROS1 and RET fusion, KRAS, and EGFR-mutational status (23-35). The typical behavior of NSCLC having one of the abovementioned alterations on 18F-FDG PET is the presence of increased SUV-derived variables, mostly SUVmax, and a prevailing metastatic pattern of initial presentation (Figure 1). Some of the specific studies shown in Table 2 may reveal contradictory results when conducted on heterogeneous cohorts, particularly with regards to EGFR mutation. Two examples are represented by the prospective trials published by Huang et al. (23) and Cho et al. (33) that report completely antithetical findings. While for the first group (23), SUVmax is significantly higher for EGFR-mutant tumors, Cho and colleagues (33) document a higher SUVmax for EGFR wild-type. A not homogenous study population can partially explain the discrepancies; while the first cohort is composed by pure ADC histology, the second study analysis a mixed population. This trend is reported also in the other retrospective studies analyzed (Table 2). While SUVmax results an independent predictor of EGFR mutation in pure ADC cohorts (29), when mixed histology is present, EGFR mutation is associated to a lower SUVmax also on multivariate analysis (25,33). The impact of SCC cases in these results appears determinant, since the squamous histotype is characterized by different metabolic and biological features, as well as prognosis, compared to ADC (36). Taken together, these findings show that FDG uptake, and consequently its association with molecular and genetic markers, should be interpreted in relation to histology. The recent investigation from Toyokawa et al. related to EZH2 hyper-expression is another example (14).
Full table
Potential impact of clinical management
Currently, specific inhibitors targeting EZH2 protein expression are under investigation (37,38). The discovery of new molecular and genetic characteristics in NSCLC can notably impact the clinical management and patient prognosis. The availability of targetable mutations and the correlation of these mutations with metabolic features might allow for a better treatment definition and response monitoring. At diagnosis, the specific sampling of more aggressive and metabolically avid tumor sites could help detect early in time non-targetable (34) or more resistant NSCLC lesions. When effective, the target drugs can determine on the course of therapy a visible reduction of tumor metabolism (39). Therefore, the possibility to investigate non-invasively the metabolic behavior of all tumor lesions, with a whole body modality such as PET/CT, might help detect early progressive or resistant lesions, which can be specifically biopsied and derived information used for further therapeutic approaches. Given the potential conflicting results on mixed cohorts, particularly with regards to some targetable mutations, the metabolic activity of NSCLC lesions on 18F-FDG PET must be used wisely and applied in a proper clinical research context, aiming to optimize the therapeutic management in accordance to tumor histology. With respect to EZH2 expression and tumor metabolism, moreover, additional data are required from prospective and multicentric studies to drive any definite conclusion.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Long Chen (Department of PET-CT Center at the Yunnan Tumor Hospital. The Third Affiliated Hospital of Kunming Medical University, Kunming, China; Department of Biochemistry and Molecular Biology of Kunming Medical University, Kunming, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.06.42). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen X, Song N, Matsumoto K, et al. High expression of trimethylated histone H3 at lysine 27 predicts better prognosis in non-small cell lung cancer. Int J Oncol 2013;43:1467-80. [Crossref] [PubMed]
- Kim W, Bird GH, Neff T, et al. Targeted disruption of the EZH2-EED complex inhibits EZH2-dependent cancer. Nat Chem Biol 2013;9:643-50. [Crossref] [PubMed]
- Lu C, Han HD, Mangala LS, et al. Regulation of tumor angiogenesis by EZH2 Cancer Cell 2010;18:185-97. [Crossref] [PubMed]
- Huqun Ishikawa R. Enhancer of zeste homolog 2 is a novel prognostic biomarker in nonsmall cell lung cancer. Cancer 2012;118:1599-606. [Crossref] [PubMed]
- Lv Y, Yuan C, Xiao X, et al. The expression and significance of the enhancer of zeste homolog 2 in lung adenocarcinoma. Oncol Rep 2012;28:147-54. [PubMed]
- Rizzo S, Hersey JM, Mellor P, et al. Ovarian cancer stem cell-like side populations are enriched following chemotherapy and overexpress EZH2. Mol Cancer Ther 2011;10:325-35. [Crossref] [PubMed]
- Cao W, Ribeiro Rde O, Liu D, et al. EZH2 promotes malignant behaviors via cell cycle dysregulation and its mRNA level associates with prognosis of patient with non-small cell lung cancer. PLoS One 2012;7:e52984 [Crossref] [PubMed]
- Geng J, Li X, Zhou Z, et al. EZH2 promotes tumor progression via regulating VEGF-A/AKT signaling in non-small cell lung cancer. Cancer Lett 2015;359:275-87. [Crossref] [PubMed]
- Riquelme E, Suraokar M, Behrens C, et al. VEGF/VEGFR-2 upregulates EZH2 expression in lung adenocarcinoma cells and EZH2 depletion enhances the response to platinum-based and VEGFR-2-targeted therapy. Clin Cancer Res 2014;20:3849-61. [Crossref] [PubMed]
- Wang X, Zhao H, Lv L, et al. Prognostic Significance of EZH2 Expression in Non-Small Cell Lung Cancer: A Meta-analysis. Sci Rep 2016;6:19239. [Crossref] [PubMed]
- Behrens C, Solis LM, Lin H, et al. EZH2 Protein Expression Associates With the Early Pathogenesis, Tumor Progression and Prognosis of Non-small Cell Lung Carcinoma. Clin Cancer Res 2013;19:6556-65. [Crossref] [PubMed]
- Xu C, Hao K, Hu H, et al. Expression of the enhancer of zeste homolog 2 in biopsy specimen predicts chemoresistance and survival in advanced non-small cell lung cancer receiving first-line platinum-based chemotherapy. Lung Cancer 2014;86:268-73. [Crossref] [PubMed]
- Liu H, Li W, Yu X, et al. EZH2-mediated Puma gene repression regulates non-small cell lung cancer cell proliferation and cisplatin-induced apoptosis. Oncotarget 2016;7:56338-54. [Crossref] [PubMed]
- Toyokawa G, Takada K, Okamoto T, et al. Elevated Metabolic Activity on 18F-FDG PET/CT Is Associated with the Expression of EZH2 in Non-small Cell Lung Cancer. Anticancer Res 2017;37:1393-401. [Crossref] [PubMed]
- Liu J, Dong M, Sun X, et al. Prognostic value of 18F-FDG PET7CT in surgical non-small cell lung cancer: a meta-analysis. PLoS One 2016;11:e0146195 [Crossref] [PubMed]
- Palsson-McDermott EM, O’Neill LAJ. The Warburg effect then and now: from cancer to inflammatory disease. Bioessays 2013;35:965-73. [Crossref] [PubMed]
- Warburg O. Metabolism of tumours. Biochem Z 1923;142:317-33.
- De Rosa V, Iommelli F, Monti M, et al. Reversal of Warburg Effect and Reactivation of Oxidative Phosphorylation by Differential Inhibition of EGFR Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:5110-20. [Crossref] [PubMed]
- Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 2010;330:1340-4. [Crossref] [PubMed]
- Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 2012;21:297-308. [Crossref] [PubMed]
- Yang W, Zheng Y, Xia Y, et al. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol 2012;14:1295-304. [Crossref] [PubMed]
- Pao W, Chmielecki J. Rational, biologically based treatment of EGFR mutant non–small cell lung cancer. Nat Rev Cancer 2010;10:760-74. [Crossref] [PubMed]
- Huang CT, Yen RF, Cheng MF, et al. Correlation of F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value and EGFR mutations in advanced lung adenocarcinoma. Med Oncol 2010;27:9-15. [Crossref] [PubMed]
- Na II, Byun BH, Kim KM, et al. 18F-FDG uptake and EGFR mutations in patients with non-small cell lung cancer: a single-institution retrospective analysis. Lung Cancer 2010;67:76-80. [Crossref] [PubMed]
- Mak RH, Diumarthy SR, Muzikansky A, et al. Role of 18F-fluorodeoxyglucose positron emission tomography in predicting epidermal growth factor receptor mutations in non-small cell lung cancer. Oncologist 2011;16:319-26. [Crossref] [PubMed]
- Choi H, Paeng JC, Kim DW, et al. Metabolic and metastatic characteristics of ALK-rearranged lung adenocarcinoma on FDG PET/CT. Lung Cancer 2013;79:242-7. [Crossref] [PubMed]
- Kaira K, Serizawa M, Koh Y, et al. Biological significance of 18F-FDG uptake on PET in patients with non-small-cell lung cancer. Lung Cancer 2014;83:197-204. [Crossref] [PubMed]
- Caicedo C, Garcia-Velloso MJ, Lozano MD, et al. Role of [18F]FDG PET in prediction of KRAS and EGFR mutation status in patients with advanced non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 2014;41:2058-65. [Crossref] [PubMed]
- Ko KH, Hsu HH, Huang T, et al. Value of 18F-FDG uptake on PET/CT and CEA level to predict epidermal growth factor receptor mutations in pulmunary adenocarcinoma. Eur J Nucl Med Mol Imaging 2014;41:1889-97. [Crossref] [PubMed]
- Yoon HJ, Sohn I, Cho JH, et al. Decoding tumor phenotypes for ALK, ROS1, and RET fusions in lung adenocarcinoma using a radiomics approach. Medicine 2015;94:e1753 [Crossref] [PubMed]
- Lee SM, Bae SK, Jung SJ, et al. FDG uptake in non-small cell lung cancer is not an independent predictor of EGFR and KRAS mutation status. Clin Nucl Med 2015;40:950-8. [Crossref] [PubMed]
- Jeong CJ, Lee HY, Han J, et al. Role of imaging biomarkers in predictive anaplastic lymphoma kinase-positive lung adenocarcinoma. Clin Nucl Med 2015;40:e34-39. [Crossref] [PubMed]
- Cho A, Hur J, Moon YW, et al. Correlation between EGFR gene mutation, cytilogic tumor markers, 18F-FDG uptake in non-small cell lung cancer. BMC Cancer 2016;16:224. [Crossref] [PubMed]
- Yoshida T, Tanaka H, Kuroda H, et al. Standardized uptake value on 18F-FDG-PET/CT is a predictor of EGFR T790M mutation status in patients with acquired resistance to EGFR-TKIs. Lung Cancer 2016;100:14-19. [Crossref] [PubMed]
- Apostolova I, Ego K, Steffen IG, et al. The asphericity of the metabolic tumour volume in NSCLC: correlation with histiopathology and molecular markers. Eur J Nucl Med Mol Imaging 2016;43:2360-73. [Crossref] [PubMed]
- Schuurbiers OC, Meijer TW, Kaanders JH, et al. Glucose metabolism in NSCLC is histology-specific and diverges the prognostic potential of 18FDG-PET for adenocarcinoma and squamous cell carcinoma. J Thorac Oncol 2014;9:1485-93. [Crossref] [PubMed]
- Hamamoto R, Saloura V, Nakamura Y. Critical roles of nonhistone protein lysine methylation in human tumorigenesis. Nat Rev Cancer 2015;15:110-24. [Crossref] [PubMed]
- Zhang H, Qi J, Reyes JM, et al. Oncogenic deregulation of EZH2 as an opportunity for targeted therapy in lung cancer. Cancer Discov 2016;6:1006-21. [Crossref] [PubMed]
- Momcilovic M, Bailey ST, Lee JT, et al. Targeted inhibition of EGFR and Glutaminase induces metabolic crisis in EFGR mutant lung cancer. Cell Reports 2017;18:601-10. [Crossref] [PubMed]


