
Microsatellite alteration in plasma DNA discriminates multiple primary lung cancer from metastatic lung cancer
Introduction
Despite recent advances in radiology and pathology, the morphologic similarity between the first and second lung tumors often complicates the differential diagnosis of multiple primary lung cancer (MPLC) and metastatic lung cancer (MLC). In addition, lung cancer patients are more vulnerable to a second lung tumor (1-3). About 0.7% to 15% of lung cancer patients suffer from MPLCs (4-8). Thus, it is crucial to establish clinical diagnosis for the selection of appropriate therapeutic management.
Studies on blood samples since 1977 revealed the level of free DNA in serum or plasma of cancer patients was higher than healthy people (9,10). Although the mechanisms underlying the presence of tumor-related cell-free DNA in blood stream are unclear, several genetic or epigenetic studies have shown the circulating DNA shares the features of tumor DNA. Mutations in oncogenes and tumor suppressor genes could be detected by extracting DNA from blood, which provides a new approach for diagnosis establishment, outcome prediction, and even investigation into the mutation status of epidermal growth factor receptor (EGFR) (10-13).
We previously analyzed several lung cancer patients whose histology included adenocarcinoma and squamous cell carcinoma. With six polymorphic microsatellite markers (D2S1363, D6S1056, D7S1824, D10S1239, D15S822, and D22S689), the “unique trend” and the “contradictory trend” represented metastatic tumors and multiple primary tumors, respectively (14-16). The “unique trend” was a consistent consequence, meaning all alleles corresponding to the six microsatellite markers were detected in primary tumor DNA, but were reduced or not observed in metastatic tumor DNA. The “contradictory trend” was an inconsistent consequence, meaning some alleles were detected in primary tumor DNA, but were reduced or not observed in metastatic tumor DNA, while other alleles were detected in metastatic tumor DNA, but were reduced or not observed in primary tumor DNA. The aim of the present study was to evaluate molecular genetic alterations in tumor cell DNA and in free circulating DNA in the blood by using the six microsatellite markers. We analyzed 12 patients with single lesion as a control group and evaluated the relationship between microsatellite alterations in tumor DNA and plasma DNA. And we examined six patients with multiple primary tumors and six patients diagnosed as pulmonary metastasis.
Methods
Patients and clinical characteristics
The single lesion group (SL group) involved 12 patients with non-small cell lung cancer (NSCLC) diagnosed between December 2013 and March 2015 at the Department of Thoracic Surgery in West China Hospital, Sichuan, China. Matched blood, tumors and normal lung tissues were collected from the SL group for molecular genetic tests. To study the role of plasma DNA compared to tumor DNA within individuals, we included 12 patients with multiple lesions group (ML group). Six of these 12 patients were diagnosed as multiple primary tumors and six patients as pulmonary metastasis according to the guideline (third edition) proposed by American College of Chest Physicians (ACCP) (17). In each patient, the first tumor was designated as Tumor 1 (Tl) and subsequent tumors as Tumor 2 (T2). Blood samples (P) were obtained before the second surgery. When multiple tumors were surgically removed from a patient simultaneously, tumor designations were assigned randomly. Matched T1, T2 and blood samples were collected from the ML group. The age at diagnosis, sex, site of tumor, histology, and tumor node metastasis (TNM) staging for each patient are given in Table 1. The TNM stage was assigned to each patient according to International Association for the Study of Lung Cancer guidelines (eighth edition) (18). The histological classification system used in this manuscript was the last version of guideline proposed by the last World Health Organization classification of lung tumors (2015) (19). The experiments were approved by the Ethics Committee of West China Hospital {No.2013 [33]}. All patients agreed to participate in the study and signed an informed consent form.
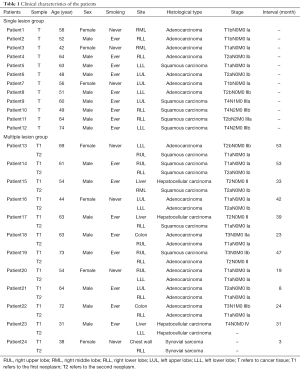
Full table
DNA isolation
Plasma was collected in ethylenediamine tetraacetic acid (EDTA)-coated tubes preoperatively and centrifuged at 4,000 rpm and 4 °C for 10 min. The plasma was carefully separated and stored at −80 °C. Tumor and normal samples were taken intraoperatively and preserved in 75% ethanol at 8 °C. For some patients, only formalin-fixed paraffin-embedded (FFPE) tissues were available. Before DNA extraction, hematoxylin-and-eosin (H&E) staining was performed to ensure a tumor cell content of at least 80%. Plasma DNA was extracted using a TIANamp micro DNA purification kit (Tiangen Biotech, Beijing, China; Ref DP316). DNA from FFPE and ethanol-preserved samples was isolated using a paraffin-embedded tissue genomic DNA extraction kit according to the manufacturer’s protocol for FFPE and non-FFPE tissues (Bioteke Corporation, Beijing, China; Ref DP7111).
Polymerase chain reaction (PCR) for microsatellite alteration analysis
Six microsatellite markers [D2S1363 (2q34), D6S1056 (6q23.2), D7S1824 (7q33), D10S1239 (10q24.3), D15S822 (15ql2), and D22S689 (22ql2.1)] which showed relatively high sensitivity in our previous study (14) were used. Information of these markers and primer sequences is available on the National Center for Biotechnology Information (NCBI) genome database (http://www.ncbi.nlm.nih.gov/). MapPairs™ primers of these 6 markers were provided by Sangon Biotech (Shanghai) Co., Ltd. For each PCR, reactions were performed in a volume of 25 µL, including 1 µL of sense primer, 1 µL of antisense primer, 1 µL of DNA, 9.5 µL of nuclease-free water and 12.5 µL of PCR Mix (2×) (PCR Master Mix 2×, Thermo Scientific). PCR cycles and electrophoresis were performed as our previous study (14,15). A positive control was included in each PCR run to check whether the reactions work and confirm negative results. We re-amplified the DNA if there were no detectable PCR products.
Results
A consecutive series of 12 patients with radically-resected primary NSCLC and 12 patients with multiple tumors were included in the study. Their clinical characteristics are presented in Table 1. The results of microsatellite analysis are summarized in Tables 2 and 3.
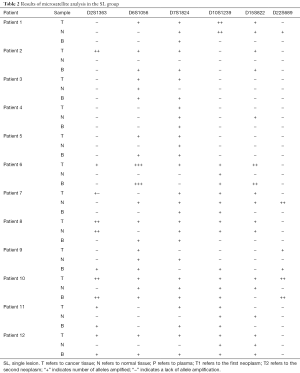
Full table
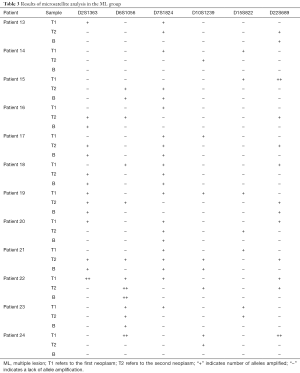
Full table
Analysis of single lesions
In the SL group, 33 (45.8%) of 72 plasma tests displayed positive results. Of the 43 corresponding tumor samples with positive results, 31 (72.1%) were also detected in plasma. In six patients (patients 1, 2, 6, 7, 8, 10), some positive loci were only detected in tumor cells. In contrast, patient 9 was detected with two positive loci (2S1363 D10S1239) only in plasma DNA (Figure 1A).
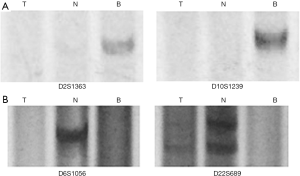
Microsatellite instability (MSI) is defined as a new band detected in tumors compared with normal tissues. MSI was detected in 11 patients (patients 1, 2, 3, 5, 6, 7, 8, 9, 10, 11, 12). There were 21 MSI events in at least one locus. From the plasma, we detected MSI in 9 (81.8%) patients (patients 2, 3, 5, 6, 8, 9, 10, 11, 12) and in 16 plasma samples (76.2% of 21 MSI events). Loss of heterozygosity (LOH) means PCR products were detected in normal lung tissues, but could not be amplified in tumors. LOH happened in 5 (41.7%) patients (patients 1, 4, 7, 9, 11). When LOH happened, plasma samples of these loci displayed negative results, which are consistent with tumor samples, regardless of normal tissues (Figure 1B).
Five of the 12 patients (patients 3, 4, 5, 11, 12) harbored identical alterations of six microsatellite loci both in plasma and tumors. These six microsatellite loci have different powers to detect positive amplification (Table 4). In D2S1363, four patients (patients 2, 6, 7, 8) showed positive results in tumors, but the PCR product was not detected in plasma. In D15S822, four patients (patients 1, 7, 8, 10) showed positive results in tumors, but the PCR product was not detectable in plasma. In D7S1824, positive results in plasma were detected in ten patients (patients 1, 2, 3, 4, 5, 7, 8, 10, 11, 12). Besides, only patient 6 displayed inconsistent result between tumor and plasma. In D22S689, two patients (9 and 10) displayed positive results in both tumor and blood, while its power to discover positive result in tumor, normal and plasma was 17%.

Full table
Analysis of multiple lesions
In microsatellite analysis of tissues, patients 13 to 22 showed the “contradictory trend” and were diagnosed as multiple primary tumors. Patients 23 and 24 showed the “unique trend” and were diagnosed as pulmonary metastasis (Table 3).
In the typical case of patient 15, hepatocellular carcinoma was pathologically diagnosed 33 months ago after a successful hepatectomy (Figure 2A). After 33 months, computerized tomography (CT) showed a mass in the right middle lobe (Figure 2B). Then the patient underwent a lobectomy and was pathologically found as squamous cell carcinoma (Figure 2C). Based on the microsatellite analysis of tumor tissues, the allelic variations of patient 15 observed at D6S1056 and D7S1824 indicate T1 could be derived from T2, which is consistent with a metastatic disease. However, the allelic variations at D15S822 and D22S689 contradict this possible lineage relationship. Thus, the collective allelic variations involving the 4 markers indicate neoplasms of patient 15 aroused independently. Consistent with the result of tissues, the result of plasma obtained before the lobectomy could represent the status of T2 (Figure 2D and Table 3).
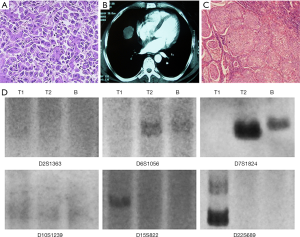
In patient 14, the alleles corresponding to D7S1824 and D15S822 were amplified from T1, but were not amplified from T2 or plasma. However, D10S1239 was detected in T2 of patient 14, but not in plasma or T1 (Figure 3). Thus, the “contradictory trend” was detected when tumor tissues were utilized. But when plasma was used to determine the linage relationship, the result indicates the two lung tumors in these two patients were related and represented metastasis disease. A similar situation was observed in patient 20.
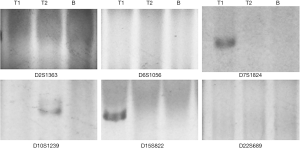
In patients 13, 16, 17, 18, 19, 21 and 22, the “contradictory trend” detected in tissues reveals the different lineage relationship between T1 and T2. Compared with tissues, the result of plasma tests might exhibit “false negative” on some alleles, indicating the alleles corresponding to the microsatellite markers were amplified from T2, but not from the plasma. However, when several microsatellite markers were combined, the “contradictory trend” between plasma and T1 was still observed.
Analysis of metastatic disease
To verify whether microsatellite PCR of plasma could detect the lineage relationship between primary and metastatic tumors, we then analyzed the patients with metastasis. Two patients were chosen, each consisting of a primary tumor and a metastatic tumor (Table 1). Lineage relationships for each of these tumor pairs were determined by molecular analysis (Table 3).
For patient 24, alleles corresponding to D6S10556 and D22S689 were detected in DNA from Tl, but not from T2 or plasma. Thus, patient 24 is a good example of clonally-related neoplasms proved by both tissue and plasma tests (Figure 4 and Table 3). Likewise, alleles corresponding to D7S1824 were observed in Tl of patient 23, but not in T2 or plasma.
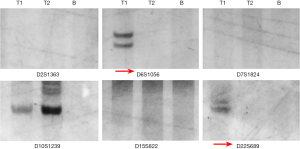
The allelic patterns between plasma and T2 were not completely identical between patients 23 and 24, who showed different allelic patterns at D15S822 and D10S1239, respectively (Figure 4 and Table 3). Alleles corresponding to these two markers obtained from plasma were negative, but the result of plasma was still a “unique trend”.
Validity evaluation of plasma DNA
If the tissue analysis was considered to be the gold standard, the sensitivity and specificity of plasma analysis in identifying multiple primary tumors are 80% (8/10) and 100% (2/2), respectively.
Discussion
When physicians establish the diagnosis of primary tumors against metastases, they largely rely on pathologic, radiologic and/or clinical findings. Studies prove the feasibility of molecular genetic analysis in investigating the lineage relationships between multiple lesions within individuals. However, these techniques are generally based on tissues.
In 2009, Mercer et al. found microsatellite alterations in tumor cell DNA could be used as a diagnostic tool to discriminate multiple primary tumors of head/neck and lung (20). Our previous studies suggest the combination of six microsatellite markers is able to discriminate tumor lineage when FFPE tissues are used (14,15). With these six markers, the “unique trend” and the “contradictory trend” represent metastatic tumors and multiple primary tumors, respectively.
However, novel diagnostic tools are required when pathologic samples are unavailable. To this end, “liquid biopsy” allows the non-invasive analysis. Compared with circulating tumor cells, circulating tumor DNA could be collected without the use of special equipment. And circulating tumor DNA reflects the characteristic of the whole population of tumors (21). Several studies have proved the feasibility of microsatellite analyses between tumor and blood (22-28). These studies, involving lung cancer, breast cancer, prostate cancer, head/neck cancer, colorectal cancer and hepatocellular carcinoma, reveal that LOH and MSI could also be detected in plasma, though the concordance varies depending on different markers (22-28). The results of our SL group reconfirm the findings from these studies. Our study shows D7S1824, D6S1056 and D10S1239 have higher positive rates both in tumor and blood, indicating the utility in detecting LOH by plasma tests. Generally, the longer amplified fragment is less likely to be detected in circulating DNA. D15S822 and D22S689 have longer fragments of 258–306 and 202–226 bps, respectively. Although D2S1363 (172–179 bps) could be amplified in 58% of tumor samples, the overall positive rate detected in plasma is relatively low. This type of DNA fragments is more likely to be degraded in blood. In some conditions (patient 9, Figure 1), however, PCR products can be detected only in plasma, which is probably because the tissue sample size is small. As the effects of intra-tumor heterogeneity, it cannot represent the exact genomic status of the whole population.
In the ML group, microsatellite analysis showed patients 13 to 22 had multiple primary tumors and patients 23 to 24 had metastatic tumors. However, when plasma samples were used, the “unique trend” was detected instead. In this condition, patients 14 and 20 were diagnosed as metastatic tumors. The alleles corresponding to D10S1239 of patient 14 and D15S822 of patient 20 exhibited “negative” in plasma tests. The reason is that the DNA fragments are too long to be amplified and might be degraded in blood. Therefore, more microsatellite markers need to be studied, especially shorter ones with higher chances of mutation. On the other hand, a combination of more than six loci could also increase the reliability. In patient 16 for example, the allelic variation of plasma at D2S1363 indicates T1 could be derived from T2, which is consistent with the analysis of metastatic tumors. However, the allelic variation of D7S1824 was noted only in T1, but not in T2 or plasma, indicating T2 could be also derived from T1. Thus, this result contradicts the possible lineage relationship of metastatic tumors. Although the allelic variations observed from plasma at D6S1056 and D22S689 were negative, diagnosis could still be established when several loci were combined. Of these 12 patients, the specificity of plasma in diagnosis establishment is 100% (2/2), so metastatic tumors are unlikely to be misdiagnosed as multiple primary tumors.
Operations for metachronous cancers provided survival that approximated the expected survival for lung cancer. Surgical intervention should be considered as a safe and effective treatment for patients with a second lung primary cancer if they satisfy the usual criteria of operability after full assessment (29,30). Lobectomy should still be considered as the treatment of choice in the management of second primary lung cancer, but sublobar resection remains a valid option in high-risk patients with limited pulmonary function (31). For stage I disease, lobectomy tends to be associated with better survival than sublobar resection, although the difference is not significant, and completion pneumonectomy is not recommended (32). Moreover, meta-analyses revealed pooled operative mortality of 7% for the second resection, pooled five-year overall survival of 46% after resection of the second NSCLC, and 79% after resection of the first NSCLC. These results suggest that surgical resection can be considered for patients who have a second primary NSCLC after resection of an initial lung cancer (33).
There are some limitations in this study. Firstly, the number of each samples involved was relatively small. Secondly, the sensitivity of the circulating plasma DNA analysis was low. Thirdly, the tumor heterogeneity might have an impact on the result of this study. Therefore, to extend the diagnostic possibility of circulating microsatellite DNA as a non-invasive follow-up tool for cancer patients, we have to study larger samples and more microsatellite markers.
Conclusions
Identical microsatellite alteration could be observed in both circulating DNA and the tumor. With polymorphic microsatellite markers, the “contradictory trend” representing multiple primary tumors that is detected in tumor cells and plasma DNA could assist in discriminating MPLC and MLC.
Acknowledgments
Funding: This study was supported by Foundation of Science and Technology Support Plan Department of Sichuan Province (2014SZ0148 and 2015SZ0158), China.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.07.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The experiments were approved by the Ethics Committee of West China Hospital {No. 2013 [33]}. All patients agreed to participate in the study and signed an informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pairolero PC, Williams DE, Bergstralh EJ, et al. Postsurgical stage I bronchogenic carcinoma: morbid implications of recurrent disease. Ann Thorac Surg 1984;38:331-8. [Crossref] [PubMed]
- Rosengart TK, Martini N, Ghosn P, et al. Multiple primary lung carcinomas: prognosis and treatment. Ann Thorac Surg 1991;52:773-8; discussion 778-9. [Crossref] [PubMed]
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9. [Crossref] [PubMed]
- Woolner LB, Fontana RS, Cortese DA, et al. Roentgenographically occult lung cancer: pathologic findings and frequency of multicentricity during a 10-year period. Mayo Clin Proc 1984;59:453-66. [Crossref] [PubMed]
- Ferguson MK, DeMeester TR, DesLauriers J, et al. Diagnosis and management of synchronous lung cancers. J Thorac Cardiovasc Surg 1985;89:378-85. [PubMed]
- Verhagen AF, van de Wal HJ, Cox AL, et al. Surgical treatment of multiple primary lung cancers. Thorac Cardiovasc Surg 1989;37:107-11. [Crossref] [PubMed]
- Lam S, MacAulay C, Palcic B. Detection and localization of early lung cancer by imaging techniques. Chest 1993;103:12S-14S. [Crossref] [PubMed]
- van Rens MT, Zanen P, Brutel de La Rivière A, et al. Survival in synchronous vs. single lung cancer: upstaging better reflects prognosis. Chest 2000;118:952-8. [Crossref] [PubMed]
- Leon SA, Shapiro B, Sklaroff DM, et al. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res 1977;37:646-50. [PubMed]
- Yoon KA, Park S, Lee SH, et al. Comparison of circulating plasma DNA levels between lung cancer patients and healthy controls. J Mol Diagn 2009;11:182-5. [Crossref] [PubMed]
- Ludovini V, Pistola L, Gregorc V, et al. Plasma DNA, microsatellite alterations, and p53 tumor mutations are associated with disease-free survival in radically resected non-small cell lung cancer patients: a study of the perugia multidisciplinary team for thoracic oncology. J Thorac Oncol 2008;3:365-73. [Crossref] [PubMed]
- Vinayanuwattikun C, Sriuranpong V, Tanasanvimon S, et al. Epithelial-specific methylation marker: a potential plasma biomarker in advanced non-small cell lung cancer. J Thorac Oncol 2011;6:1818-25. [Crossref] [PubMed]
- Akca H, Demiray A, Yaren A, et al. Utility of serum DNA and pyrosequencing for the detection of EGFR mutations in non-small cell lung cancer. Cancer Genet 2013;206:73-80. [Crossref] [PubMed]
- Shen C, Xu H, Liu L, et al. "Unique trend" and "contradictory trend" in discrimination of primary synchronous lung cancer and metastatic lung cancer. BMC Cancer 2013;13:467. [Crossref] [PubMed]
- Shen C, Wang X, Tian L, et al. "Different trend" in multiple primary lung cancer and intrapulmonary metastasis. Eur J Med Res 2015;20:17. [Crossref] [PubMed]
- Shen C, Wang X, Tian L, et al. Microsatellite alteration in multiple primary lung cancer. J Thorac Dis 2014;6:1499-505. [PubMed]
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-e399S.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Mercer RR, Lucas NC, Simmons AN, et al. Molecular discrimination of multiple primary versus metastatic squamous cell cancers of the head/neck and lung. Exp Mol Pathol 2009;86:1-9. [Crossref] [PubMed]
- Heitzer E, Auer M, Ulz P, et al. Circulating tumor cells and DNA as liquid biopsies. Genome Med 2013;5:73. [Crossref] [PubMed]
- Kakimoto Y, Yamamoto N, Shibahara T. Microsatellite analysis of serum DNA in patients with oral squamous cell carcinoma. Oncol Rep 2008;20:1195-200. [PubMed]
- Müller I, Beeger C, Alix-Panabières C, et al. Identification of loss of heterozygosity on circulating free DNA in peripheral blood of prostate cancer patients: potential and technical improvements. Clin Chem 2008;54:688-96. [Crossref] [PubMed]
- Pang JZ, Qin LX, Wang QQ, et al. Loss of heterozygosity of plasma circulating DNA from hepatocellular carcinoma patients and its clinical significance. Zhonghua Gan Zang Bing Za Zhi 2007;15:906-9. [PubMed]
- Schwarzenbach H, Chun FK, Müller I, et al. Microsatellite analysis of allelic imbalance in tumour and blood from patients with prostate cancer. BJU Int 2008;102:253-8. [Crossref] [PubMed]
- Sozzi G, Conte D, Mariani L, et al. Analysis of circulating tumor DNA in plasma at diagnosis and during follow-up of lung cancer patients. Cancer Res 2001;61:4675-8. [PubMed]
- Beau-Faller M, Gaub MP, Schneider A, et al. Plasma DNA microsatellite panel as sensitive and tumor-specific marker in lung cancer patients. Int J Cancer 2003;105:361-70. [Crossref] [PubMed]
- Bruhn N, Beinert T, Oehm C, et al. Detection of microsatellite alterations in the DNA isolated from tumor cells and from plasma DNA of patients with lung cancer. Ann N Y Acad Sci 2000;906:72-82. [Crossref] [PubMed]
- Battafarano RJ, Force SD, Meyers BF, et al. Benefits of resection for metachronous lung cancer. J Thorac Cardiovasc Surg 2004;127:836-42. [Crossref] [PubMed]
- Aziz TM, Saad RA, Glasser J, et al. The management of second primary lung cancers. A single centre experience in 15 years. Eur J Cardiothorac Surg 2002;21:527-33. [Crossref] [PubMed]
- Zuin A, Andriolo LG, Marulli G, et al. Is lobectomy really more effective than sublobar resection in the surgical treatment of second primary lung cancer? Eur J Cardiothorac Surg 2013;44:e120-5; discussion e125.
- Yang J, Liu M, Fan J, et al. Surgical treatment of metachronous second primary lung cancer. Ann Thorac Surg 2014;98:1192-8. [Crossref] [PubMed]
- Hamaji M, Ali SO, Burt BM. A meta-analysis of resected metachronous second non-small cell lung cancer. Ann Thorac Surg 2015;99:1470-8. [Crossref] [PubMed]


