
Survival outcomes of surgical resection of metastasis to the breast from extra-mammary malignancies: an individual patient data analysis
Introduction
Secondary neoplasms of the breast comprised 3% of the breast tumors and the majority were metastases from the contralateral breast (1-3). Non-hematological metastasis to the breast was approximately 0.3–0.4% of contemporary malignant mammary tumors (1,3,4). It remains controversial whether surgical resection of either the primary tumor or the metastatic site would improve patients’ survival outcomes. Retrospective and population-based studies had shown that primary surgery would be associated with improved survival in stage IV breast cancer, in asymptomatic colorectal cancer and even in male stage IV breast (5-8). In prospective randomized trials, primary surgery might be beneficial in selective patients with conflicting evidence.
Surgical resection of liver metastatic lesions has been widely accepted for with liver metastases from colorectal cancer and neuro-endocrine tumors (9,10). Hepatic resection for liver metastases from breast cancer before progression of disease even with chemotherapy might result in better outcomes of selected patients or allow time off from systemic chemotherapy (11,12). However, it remains unclear whether the surgical resection of metastasis to the breast from extra-mammary malignancies would improve survival outcome.
Methods
Patients and clinicopathological characteristics
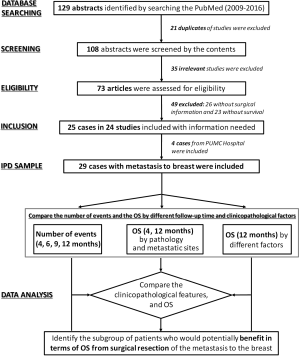
The individual patient data (IPD) meta-analysis was performed according to the method previously reported (13,14). There were 129 abstracts identified during 2009–2016 by searching the PubMed by searching with keywords such as “metastasis to the breast”, “extra-mammary malignancy”, “in breast metastasis”, “secondary neoplasms of the breast” and “extra-mammary cancer metastatic to the breast”. Twenty-one duplicates were excluded after screening by the content of abstracts. Thirty-five irrelevant studies were excluded after eligibility evaluation, and 49 studies without therapeutic or survival information were also excluded. There were 25 cases reported by 24 studies and 4 previously unreported cases from PUMC Hospital (altogether 29 cases) included. The clinicopathological characteristics and overall survival (OS) by 4 and 12 months were compared respectively among all patients and several subgroups of patients. Patients who might potentially benefit from surgical resection of the metastatic lesion to the breast were identified (Figure 1).
Statistical analysis
The OS time was defined as the time from the date of diagnosis of breast metastasis from extra-mammary malignancy to death. OS were analyzed by the Kaplan-Meier curve method, and were compared by means of the log rank test for all patients and each subgroup. The significance threshold was set at P<0.05. All analyses were conducted using SPSS software, version 18.0 (SPSS, Inc. Chicago, IL, USA)
Results
Descriptive information of the study cohort
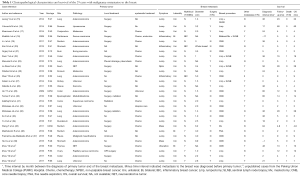
There were 25 cases reported by 24 studies and 4 previously unreported cases from PUMC Hospital (altogether 29 cases) included in this IPD meta-analysis (Figure 1 and Table 1). The breast symptoms included 22 lumps (75.9%), 4 huge mass (13.8%), 3 inflammation (10.3%), 1 ulceration (3.4%) and 1 non-palpable lesion (3.4%). There were 2 male patients (6.9%) with breast metastasis and 3 patients with bilateral breast metastasis (10.3%). The primary malignancies included 10 gastrointestinal tract (34.5%), 7 lung (24.1%), 4 urogenital (13.8%) and 3 trunk and limbs (10.3%) and adenocarcinoma (48.3%) was the commonest primary pathology. There were 12 patients (41.4%) with breast as the only distant metastases. Seven (24.1%) patients’ breast metastases were diagnosed simultaneously with the primary malignancies, and 2 (6.9%) breast metastases were detected before the primary. Sixteen patients (55.2%) received surgical resection of the in-breast metastases, including 14 extended lumpectomies, 2 mastectomies and 5 sentinel lymph node biopsies.
Full table
Comparison of OS among all patients and subgroups of patients
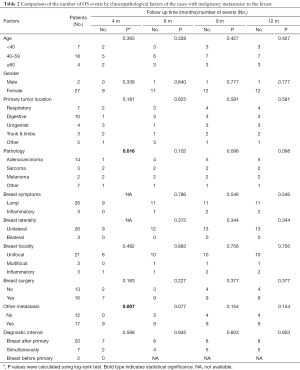
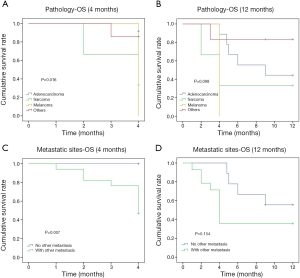
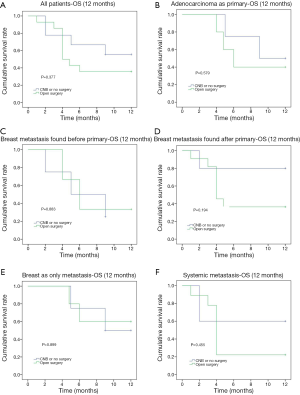
The 12-month OS rate was 55.2% and the median survival was 5 months (1–60 months). The comparison of OS events by 4, 6, 9 and 12 months among different subgroups of patients showed that the 4-month OS events were different among different pathology of the primary cancer (P=0.016) and between whether there were other extra-mammary metastases (P=0.007) (Table 2). Patients with primary adenocarcinoma and with only in-breast metastasis showed less OS events compared to patients with sarcoma, melanoma or other primary cancer pathology and to patients with systemic metastases during the first 4 months (Figure 2). However, there was no significant difference between these two subgroups of patients if the comparison was performed by 12 months (Figure 2). The comparison of 12-month OS between patients received surgical resection of the in-breast metastatic lesion and those without surgery showed no significant difference in survival outcomes among all patients and subgroups of patients, regardless of whether the in-breast metastasis was found before or after the primary malignancy, and of whether breast was the only distant metastasis site (Figure 3).
Full table
Discussion
Extramammary non-hematological metastasis to the breast comprised 0.3–0.4% of malignant breast tumors (1,4). On one hand, surgical resection of primary tumor of metastatic breast cancer is associated with favorable outcomes among selective patients in prospective randomized trials as well as in retrospective and population-based studies (5,7,8). On the other hand, surgical resection of liver, lung and even bran metastatic lesion might also be beneficial in certain primary cancers and in selective patients (9,10,39,40). It remained unclear whether surgical management of in-breast metastasis from extra-mammary malignancies would improve survival outcome.
The commonest primary malignancy site developing in-breast metastases was the gastrointestinal tract (34.5%) with lung (24.1%) as the second, while the commonest pathology of primary was adenocarcinoma (48.3%). A large series of intramammary metastases of 280 cases from Abbas et al. showed that the three commonest primary malignancies as melanoma (17.5%), ovarian carcinoma (14.6%) and lung cancer (14.3%) (3), while another large series of 169 cases from Williams et al. showed as skin (39.6%), respiratory (24.3%) and gynecological (14.2%) cancers (41). The metastases to the breast could either be multifocal (10.3%) or be bilateral cancers (10.3%). Interestingly, there were 2 males colorectal cancer patients with metastasis to the breast, which was also reported by Zhou et al. and Luo et al. (2,42). Because in-breast metastasis might be misdiagnosed with breast cancer especially when it was found before the primary malignancy, as 2 cases (6.9%) were reported in this study and one of them underwent bilateral mastectomies + sentinel lymph node biopsies (18,38). In 12–31% of cases metastases to the breast they have been reported as the first finding leading to a diagnosis of an extramammary cancer.
The median OS of metastases to the breast from extramammary solid tumors was 9.2–24 months (2,41,42). Study from Williams et al. showed better survival among patients who underwent surgical resection for in-breast metastases (41). In our study the 12-month OS rate was 55.2%, and the median survival was 5 month (1–60 months). In all patients as well as in all subgroups of patients, surgical resection of the metastatic lesion in the breast could achieve similar prognostic outcome in terms of 12-month OS. Considering breast local symptom such as huge mass (13.8%), inflammation (10.3%) and ulceration (3.4%) might compromise the patients’ quality of life, surgical management could potentially improve selective patients’ quality of life and achieve 12-month OS non-inferior to those patients without surgery.
Our study had several limitations. Firstly, the PubMed database searching might not find all studies suitable for this IPD analysis. Secondly, it was a retrospective IPD study with very limited number of cases and short follow-up time. Since the individual patient’s surgical and survival information from large case series was usually unavailable, cases included in this IPD analysis were usually from case reports, resulting in selection bias. Thirdly, patients included in this study were from all over the world thus might be treated quite differently and the OS was acquired from different studies instead of direct follow-up of the patients. Fourthly, also due to the limited number of included cases, Cox analysis could not be performed to identify the OS related prognostic factors.
In conclusion, surgical resection of metastases to the breast from extra-mammary malignancies could achieve similar survival outcome compared to patients who only received core needle biopsy, with potential of improving the quality of life for patients with breast ulceration, huge mass and inflammatory changes.
Acknowledgments
We would like to thank all of the patients for their participation in this study.
Funding: This work was supported by the Natural Science Foundation of China (Grant No. 81001183), the Science & Technology Research Project of Returned Visiting Scholar, Ministry of Human Resources and Social Security [2015] and the Twelfth Five Year Key Programs for Science and Technology Development of China (Grant No. 2014BAI028B03).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.08.18). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Peking Union Medical College Hospital, Chinese Academy of Medical Sciences. Since this is a retrospective study without any intervention or treatment to patients, the Ethics Committee Board just approved it without giving an ID. Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Georgiannos SN, Chin J, Goode AW, et al. Secondary neoplasms of the breast: a survey of the 20th Century. Cancer 2001;92:2259-66. [PubMed]
- Zhou S, Yu B, Cheng Y, et al. Metastases to the breast from non-mammary malignancies: a clinicopathologic study of 28 cases. Zhonghua Bing Li Xue Za Zhi 2014;43:231-5. [PubMed]
- Abbas J, Wienke A, Spielmann RP, et al. Intramammary metastases: comparison of mammographic and ultrasound features. Eur J Radiol 2013;82:1423-30. [PubMed]
- Lee AH. The histological diagnosis of metastases to the breast from extramammary malignancies. J Clin Pathol 2007;60:1333-41. [PubMed]
- Blanchard DK, Shetty PB, Hilsenbeck SG, et al. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg 2008;247:732-8. [PubMed]
- Ahmed S, Fields A, Pahwa P, et al. Surgical Resection of Primary Tumor in Asymptomatic or Minimally Symptomatic Patients With Stage IV Colorectal Cancer: A Canadian Province Experience. Clin Colorectal Cancer 2015;14:e41-7. [PubMed]
- Warschkow R, Guller U, Tarantino I, et al. Improved Survival After Primary Tumor Surgery in Metastatic Breast Cancer: A Propensity-adjusted, Population-based SEER Trend Analysis. Ann Surg 2016;263:1188-98. [PubMed]
- Thomas A, Khan SA, Chrischilles EA, et al. Initial Surgery and Survival in Stage IV Breast Cancer in the United States, 1988-2011. JAMA Surg 2016;151:424-31. [PubMed]
- Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125-35. [PubMed]
- Mayo SC, de Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol 2010;17:3129-36. [PubMed]
- Sadot E, Lee SY, Sofocleous CT, et al. Hepatic Resection or Ablation for Isolated Breast Cancer Liver Metastasis: A Case-control Study With Comparison to Medically Treated Patients. Ann Surg 2016;264:147-54. [PubMed]
- Takemura N, Saiura A. Role of surgical resection for non-colorectal non-neuroendocrine liver metastases. World J Hepatol 2017;9:242-51. [PubMed]
- Pietschmann S, von Bueren AO, Henke G, et al. An individual patient data meta-analysis on characteristics, treatments and outcomes of the glioblastoma/gliosarcoma patients with central nervous system metastases reported in literature until 2013. J Neurooncol 2014;120:451-7. [PubMed]
- Anghileri E, Castiglione M, Nunziata R, et al. Extraneural metastases in glioblastoma patients: two cases with YKL-40-positive glioblastomas and a meta-analysis of the literature. Neurosurg Rev 2016;39:37-45; discussion -6.
- Jeong YJ, Bong JG, Oh HK, et al. Metachronous isolated breast metastasis from pulmonary adenocarcinoma with micropapillary component causing diagnostic challenges. BMC Cancer 2014;14:736. [PubMed]
- Yokouchi M, Nagano S, Kijima Y, et al. Solitary breast metastasis from myxoid liposarcoma. BMC Cancer 2014;14:482. [PubMed]
- Kharmoum S, Mohamed M, Benhammane H, et al. Conjonctival melanoma metastatic to the breast: a case report. BMC Res Notes 2014;7:621. [PubMed]
- Khalifeh I, Deavers MT, Cristofanilli M, et al. Primary peritoneal serous carcinoma presenting as inflammatory breast cancer. Breast J 2009;15:176-81. [PubMed]
- Li J, Fang Y, Li A, et al. Breast metastases from rectal carcinoma. Chin Med J (Engl) 2011;124:1267-9. [PubMed]
- Kim SJ. Magnetic resonance imaging features of inflammatory breast metastasis from gastric signet-ring cell carcinoma. Clin Imaging 2013;37:569-73. [PubMed]
- Orgüç S, Başara I, Poçan T, et al. Ewing sarcoma metastasis to breast. Diagn Interv Radiol 2012;18:167-70. [PubMed]
- Perin T, Canzonieri V, Memeo L, et al. Breast metastasis of primary colon cancer with micrometastasis in the axillary sentinel node: a metastasis that metastasized? Diagn Pathol 2011;6:45. [PubMed]
- Maounis N, Chorti M, Legaki S, et al. Metastasis to the breast from an adenocarcinoma of the lung with extensive micropapillary component: a case report and review of the literature. Diagn Pathol 2010;5:82. [PubMed]
- La Rosa S, Casnedi S, Maragliano R, et al. Breast Metastasis as the First Clinical Manifestation of Ileal Neuroendocrine Tumor. A Challenging Diagnosis with Relevant Clinical Implications. Endocr Pathol 2015;26:145-51. [PubMed]
- Wilsher MJ, Moncrieff NJ. Melanoma metastatic to a ruptured silicone breast implant capsule. Indian J Pathol Microbiol 2012;55:86-8. [PubMed]
- Shen YW, Sui YX, Zhang XM, et al. Ipsilateral breast metastasis from a pulmonary adenocarcinoma: a case report and a focused review of the literature. Int J Clin Exp Pathol 2015;8:9647-54. [PubMed]
- Solaini L, Bianchi A, Filippini L, et al. A Mammary Nodule Mimicking Breast Cancer. Int Surg 2014;99:200-2. [PubMed]
- Sibartie S, Larkin JO, Lee G, et al. Metastatic uterine leiomyosarcoma presenting as a breast lump. Ir J Med Sci 2011;180:889-91. [PubMed]
- Ho YY, Lee WK. Metastasis to the breast from an adenocarcinoma of the colon. J Clin Ultrasound 2009;37:239-41. [PubMed]
- Ternier F, Hadjaj D, Jacquemier J. Sonographic appearance of a metastasis to the breast from a cerebellar medulloblastoma. J Clin Ultrasound 2010;38:335-7. [PubMed]
- Aitelhaj M, Khoyaali SL, Boukir A, et al. Breast and splenic metastases of squamous cell carcinoma from the uterine cervix: a case report. J Med Case Rep 2014;8:359. [PubMed]
- Mirrielees JA, Kapur JH, Szalkucki LM, et al. Metastasis of primary lung carcinoma to the breast: a systematic review of the literature. J Surg Res 2014;188:419-31. [PubMed]
- Ko K, Ro JY, Hong EK, et al. Micropapillary Lung Cancer with Breast Metastasis Simulating Primary Breast Cancer due to Architectural Distortion on Images. Korean J Radiol 2012;13:249. [PubMed]
- Yu H, Song H, Jiang Y. Duodenal Adenocarcinoma Metastatic to the Breast. Medicine 2016;95:e3088 [PubMed]
- Wang T, Lv Y-G, Yan Q-G, et al. Rectal Carcinoma Metastatic to the Male Breast after 7 Years Case Report. Onkologie 2011;34:544-6. [PubMed]
- Makhdoomi R, Mustafa F, Ahmad R, et al. Bilateral breast metastasis from mucinous adenocarcinoma of the rectum: a case report and review of the literature. Turk Patoloji Derg 2013;29:231-4. [PubMed]
- Framarino-dei-Malatesta M, Sammartino P, Derme M, et al. Breast cancer or metastasis? An unusual case of metastatic malignant pleural mesothelioma to the breast. World J Surg Oncol 2015;13:79. [PubMed]
- He CL, Chen P, Xia BL, et al. Breast metastasis of gastric signet-ring cell carcinoma: a case report and literature review. World J Surg Oncol 2015;13:120. [PubMed]
- Hacker NF, Rao A. Surgical management of lung, liver and brain metastases from gynecological cancers: a literature review. Gynecol Oncol Res Pract 2016;3:7. [PubMed]
- Shen CJ, Lim M, Kleinberg LR. Controversies in the Therapy of Brain Metastases: Shifting Paradigms in an Era of Effective Systemic Therapy and Longer-Term Survivorship. Curr Treat Options Oncol 2016;17:46. [PubMed]
- Williams SA, Ehlers RA 2nd, Hunt KK, et al. Metastases to the breast from nonbreast solid neoplasms: presentation and determinants of survival. Cancer 2007;110:731-7. [PubMed]
- Luo Y, Xu B, Li Q, et al. Clinicopathological features and prognosis of metastases to the breast from extramammary solid tumors. Zhonghua Zhong Liu Za Zhi 2014;36:453-6. [PubMed]


