
A clinical comparison of short-term efficacy, survival and adverse reactions between raltitrexed/cisplatin-based and docetaxel/cisplatin-based concurrent chemoradiotherapy in the treatment of advanced esophageal squamous cell carcinoma
Introduction
Esophageal cancer is one of most common malignancies worldwide with an increasing incidence. According to the latest global cancer data from the World Health Organization (WHO) (1), there were newly-diagnosed cases of 455,800 and disease related deaths of 400,200 in 2012. Esophageal cancer is usually 3 to 4 times more common among men than women (1). It ranks as the second most common cause of cancer-related mortality. In Chinese population, the epidemiology of esophageal cancer shows special features: it is the fourth-fifth incident cancer and the third-fourth leading cause of cancer death; the mortality rate ranks first in the world (2,3). The largest proportion of new esophageal cancer cases are diagnosed among those between ages 60 and 74 years, followed by those between ages 45 and 59 years (3). Moreover, 90% cases are diagnosed as squamous cell carcinoma based on pathology. Because esophageal cancer has no specific clinical manifestations at the early stage, most detected cases have already been in the advanced stage and un-resectable or medically unfit for surgery. And there are some cases unwilling to accept surgical resection. Non-surgical treatments, such as concurrent chemoradiotherapy, are still the main options for advanced victims, but the overall 5-year survival rate is poor, about 10% to 20%. Although concurrent chemoradiotherapy is now accepted as a standard care for advanced lesions, a considerable number of patients may develop intolerable adverse reactions. A safe and effective concurrent regimen, as an urgent demand for the treatment of esophageal cancer, has been under investigation.
Raltitrexed, a quinazoline folate analogue, is a new generation of aqueous soluble thymidylate synthase inhibitor. Previous data showed that the effect of radiation sensitization of raltitrexed is not inferior to 5-fluorouracil (5-Fu) in vitro (4). The regent has been used in the treatment of multiple solid tumors, such as advanced colorectal cancer (5,6) and cervical carcinoma (7). In present study, we, using docetaxel/cisplatin based concurrent chemoradiotherapy as the control, evaluated the short-term efficacy and toxic reactions of raltitrexed/cisplatin-based concurrent radiotherapy in the treatment of advanced esophageal carcinoma.
Methods
Patients
From January 2013 to June 2016, 104 untreated patients with esophageal squamous cell carcinoma (ESCC) were enrolled in this study. They had a median age of 66 years (range, 37–79 years). The enrolled patients had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2 and had not serious medical diseases. Their expected survivals were estimated to be more than 3 months. The laboratory examination requirements for all enrolled patients included: neutrophil ≥1.5×109/L, hemoglobin ≥100 g/L, platelet ≥100×109/L, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) values fewer than 2 times the upper limit of the normal value (ULN), and serum creatinine less than 1.5 times ULN. The exclusion criteria included: clinical stages of T1N0M0, distant metastasis, esophageal fistula or perforation, cachexia and poor general condition (ECOG score greater than 2 points). They were randomly divided into raltitrexed group and docetaxel group, and their baseline characteristics were shown in Table 1.
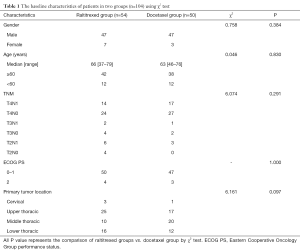
Full table
Radiotherapy procedure
All patients were subjected to intensity-modulated radiation therapy (IMRT). Radiation plans were generated using Pinnacle Version 3.0. CT-based simulation localization was performed prior to the intervention. The gross tumor volume (GTV), clinical target volume (CTV) and planned target volume (PTV) were determined based on the ICRU50 and ICRU62 reports. IMRT or 3DCRT was delivered using 6-MV X-rays (Radiotherapy machine: Oncor Liner Accelerator). A fractional daily dose of 2.0 Gy (5 fractions per week) was prescribed. The total dose ranged 60 to 64 Gy. PTV 95% to receive a prescription dose of 100% was required. The dose to the organs at risk was constrained as follows: the both lungs dose was limited to V20 <30% and a mean dose less than 13 Gy. The heart dose was limited to V30 ≤40% and V40 ≤30%. Spinal cord maximum dose was held to 45 Gy. Radiotherapy plans were carried out after verification. All patients completed radiotherapy procedure successfully.
Concurrent chemotherapy procedure
Chemotherapy procedure was conducted for two cycles concomitantly with radiation. On day 1 and 22, patients received intravenous administration of raltitrexed at a dose of 2.5 mg/m2 for more than 15 minutes, or a dose of 60 mg/m2 of docetaxel for 1 hour, following intravenous administration of cisplatin at a dose of 75 mg/m2 for 2–6 hours. At the second cycle, timing or dose of agents would be adjusted according to patients’ responses to the first cycle. For example, the dose of all agents was reduced by 25% if there was grade 3 hematological toxicity. Chemotherapy procedure would be canceled if patients developed grade 4 hematological toxicity.
Endpoints and follow-up
The primary endpoints were 1-year survival rate, local progression free survival rate, progression free survival rate. The secondary endpoints were objective effective rate, DCR and adverse events. According to the criteria for Response Evaluation Criteria In Solid Tumors (RECIST, 1.1 Edition), the short-term responses were defined as complete response (CR), partial response (PR), stable disease (SD) and disease progression (PD). Response rate (RR) was calculated with CR+PR, and the DCR was defined as CR + PR + SD. Toxicities were evaluated according to the NCI-CTC (4.0 version), which divides the toxicities into grades 0–4. Follow-up was conducted one month after the completion of treatment, and then regularly at 3-monthly intervals in the first 2 years and at 6-monthly intervals after 2 years. At the time of follow-up, all patients received chest and abdomen CT scan and esophageal barium meal examination. Electronic gastroscopy examination was carried out at 6-monthly intervals. The median follow-up time was 13.5 months (range, 3 to 35 months), and the follow-up rate was 97.1%. The overall survival (OS) was defined as the time from start of treatment to loss of follow-up, death or last follow-up. Progression-free survival was defined as the time from start of treatment to tumor progression (recurrence and/or metastasis) or death; Survival time is referred to as the time from the beginning of treatment to tumor recurrence within the radiation field or death.
Statistical analysis
All of the statistical analyses were performed using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA). Count data were processed by chi square test (including continuous correction test or Fisher’s exact test). Based on survival data of follow up, survival curve were drawn using Kaplan-Meier method (Log-rank test used for comparison of two groups). P value of 0.05 or less was considered to indicate statistical significance. The patient who did not have deaths, progression, or local progression, were defined as censored at the time of follow-up.
Results
Short-term efficacy
All 104 patients had completed evaluation for efficacy. In the raltitrexed/cisplatin group, 43 cases were CR, 3 cases PR, 5 cases SD, and PD 3 cases. Their RR and DCR were 85.2% and 94.4%, respectively; in the docetaxel/cisplatin group, 36 cases were CR, 4 cases PR, 6 cases SD, and PD 4 cases. Their RR and DCR were 80% and 92%, respectively. There was no statistically significant difference in RR (χ2=0.488, P=0.485) and DCR (P=0.708) between the two groups.
Survival
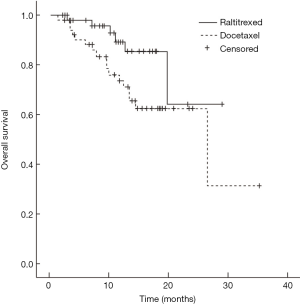
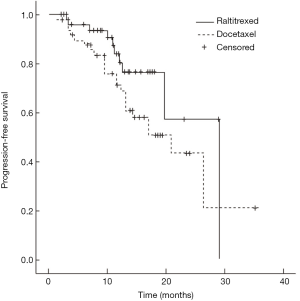
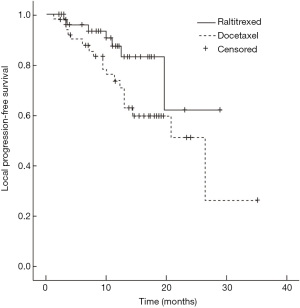
Median survival time of the raltitrexed group has not reached and the 1-year survival rate was expected to be 85.1%; the median survival time of docetaxel group was 26.4 months (95% CI: 9.5–43.3 months), and their 1-year survival rate was expected to be 71.0%; Log-rank test showed a statistically significant difference in the total survival time between two groups (χ2=4.181, P=0.041) (Figure 1). In raltitrexed group the median progression free survival time was 29.1 months, and 1-year progression free survival rate was estimated to be 80.4%; in the docetaxel group, the median progression free survival time was 20.8 months (95% CI: 12.4–29.2 months), and the 1-year progression free survival rates was expected to be 68.6%. There were no significant differences between the two groups (χ2=2.931, P=0.087) (Figure 2). Patients in the raltitrexed group did not achieve the median progression free survival time. Their 1-year progression free survival rate was estimated to be 83.2%; the median local progression free survival time in the docetaxel was 26.4 months (95% CI: 13.4–39.4 months), the local progression free survival rate was estimated to be 71.0%; The OS time was statistically different between two groups (χ2=4.063, P=0.044) (Figure 3).
Safety
All patients had completed the treatment successfully. The main adverse events associated with treatment were leukopenia, thrombocytopenia, gastrointestinal reactions, elevated alanine aminotransferase and aspartate aminotransferase, radiation esophagitis and radiation pneumonia. Through the appropriate treatments, the vast majority of adverse reactions were improved. Esophageal stenosis occurred in 2 patients in the raltitrexed group and in 3 patients in the docetaxel group. In terms with incidence of the main adverse reactions, there was no statistically significant difference between the two groups (Table 2).
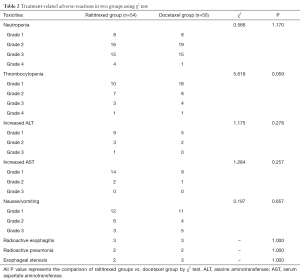
Full table
Discussion
Concurrent chemoradiotherapy has become one of the standard treatments for patients who are unwilling to accept surgical resection of esophageal cancer or have the incurable advanced lesions. The result of a prospective randomized trial (Radiation Therapy Oncology Group 8501) has shown that the 5-FU plus cisplatin-based concurrent chemoradiotherapy was able to significantly increase local recurrence rate and OS, compared with radiotherapy alone (8). This regimen has been used widely in clinical practice. However, its adverse effects were significantly more frequent and serious than those of radiotherapy alone. Some patients have to delay or discontinue their treatment because of failing to tolerate adverse effects, thus affecting the curative effect and long-term survival. Additionally, administration of 5-FU is inconvenient due to continuous intravenous infusion for 24 hours. Most patients with advanced esophageal cancer are older in age. For these above-mentioned reasons, 5-FU-combined chemotherapy is not easy to be tolerance by patients. 5-FU-induced cardiotoxicity has also been paid more and more attention in clinical practice recently (9). These cardiotoxicity effects, including acute coronary syndromes, cardiomyopathy, malignant arrhythmia vasospastic angina and sudden cardiac death, are infrequent but fatal. So it is still urgent for oncologists to find a convenient concurrent chemoradiotherapy with high efficiency and low toxicity. Many oncologists have made an effort to use alternative drugs or regimens, especially taxane base ones. For example, some clinical studies have shown that paclitaxel or docetaxel -based combination with cisplatin has become a good alternative treatment, which demonstrates higher efficacy and more favorable overall toxicity (10).
Raltitrexed is a specific inhibitor of thymidylate synthase, which could undergo intracellular uptake through the reduced folate carrier and instantly metabolized to a series of polyglutamic acid derivatives by folylpolyglutamate synthetase. These metabolites have more potent inhibitory activities against thymidylate synthase, and their intracellular retention behaviors are associated with long-term cytotoxicity. The actions of raltitrexed don’t need folic acid calcium cooperation and therefore, doesn’t further increase mucosal toxicity. Compared with 5-Fu, the agent is characterized by well-established efficacy, no cardio toxicity, convenience of administration and favorable acceptability. The agent is mainly used for the treatment of advanced colorectal cancer (11), but some basic and clinical experiments have suggested that its anti-tumor spectrum may be wider. The combination of raltitrexed and radiotherapy has been used for the treatment of colorectal cancer (5,6), cervical cancer (7) and other tumors. Therapeutic results showed its favorable tolerability. Moreover, it, together with preoperative concurrent radiotherapy, could improve pathological remission rate and significantly reduce tumor stage (6). However, there are few researches on raltitrexed-based concurrent chemoradiotherapy in esophageal carcinoma. Cisplatin is one of basic agents in the treatment of esophageal carcinoma. In addition to its own anti-tumor effects, cisplatin is able to increase concurrent radiotherapy effects. Therefore, this study, using docetaxel/cisplatin-based concurrent chemoradiotherapy as the control, evaluated the effects of raltitrexed/cisplatin concurrent IMRT or 3D-CRT for the treatment of advanced esophageal cancer.
In the present study, our results showed that RR and DCR of patients who underwent concurrent raltitrexed/cisplatin-based chemoradiotherapy have achieved 85% and 94.4%, respectively. Their median progression-free survival time was 29.1 months, and their 1-year progression-free survival rate and 1-year survival rate were expected to be 85.1% and 85.1%, respectively. There were no significant differences in RR, DCR and 1-year progression-free survival rate between raltitrexed/cisplatin-based concurrent chemoradiotherapy and control group. However, the 1-year survival rate and 1-year local progression-free survival rate in raltitrexed/cisplatin group were expected to be significantly higher than that in the control group. In comparison with the RTOG 85-01 study, the RR and 1-year survival rate were higher in both groups, which may be contributed to by increased radiotherapy dose, modified radiotherapy technology and earlier stag of some patients. Although short period of follow-up was the main drawback of this study, the preliminary results showed that the short-term effects of raltitrexed/cisplatin based concurrent radiotherapy were similar to that of docetaxel/cisplatin with the higher year survival rate and 1-year local progression-free survival rate. We expect that the long-term survival efficacies should also be favorable, which needs further evaluation and follow-up.
The major adverse events in both groups were neutropenia, thrombocytopenia gastrointestinal reactions, elevated transaminase, radiation esophagitis and radiation pneumonia. Most of adverse events were alleviated through active interventions and therefore didn’t affect the concurrent chemoradiotherapy process. The incidence of adverse reactions was not significantly different between the two groups. Some researchers have reported that the incidence of raltitrexed-induced cardio toxicity, especially fatal cardiac complications, was significantly lower than that of 5-FU. So they recommended raltitrexed as an alternative agent for patients who has a history of serious heart disease or is not suitable for 5-FU due to a risk of heart disease development (12,13). Ransom et al. also found that 5-FU caused cardiac damage were not exaggerated after replacement of 5-FU with raltitrexed (14). In the present study, no patients developed cardiac complications, which further confirmed the safety of raltitrexed in terms of cardiotoxicity.
In conclusion, our study found that raltitrexed/cisplatin-based concurrent chemoradiotherapy is an effective treatment regimen for the treatment of esophageal carcinoma. Its short-term efficacies were similar to that of docetaxel/cisplatin concurrent chemoradiotherapy with a higher 1-year survival rate and 1-year local progression-free survival rate. Because of good tolerance, better safety and ease of use, this regimen is worth being further evaluated through large sample, multi-center phase III clinical studies.
Acknowledgments
We would like to express our special thanks to Dr. Hong-Cheng Zhu for his support of language and format guide in this article.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2017.07.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This research has been approved by the ethics committee of Nanjing Bayi Hospital (approval number: 81YY-KYLL-13-03). Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Chen W, Zheng R, Zhang S, et al. Report of Cancer Incidence and Mortality in China, 2012. Zhongguo Zhong Liu 2016;25:1-8. (in Chinese).
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Teicher BA, Ara G, Chen YN, et al. Interaction of tomudex with radiation in vitro and in vivo. Int J Oncol 1998;13:437-42. [PubMed]
- Picardi V, Deodato F, Guido A, et al. Concurrent Chemoradiation with Concomitant Boost in Locally Advanced Rectal Cancer: A Phase II Study. Anticancer Res 2016;36:4081-7. [PubMed]
- Avallone A, Delrio P, Pecori B, et al. Oxaliplatin plus dual inhibition of thymidilate synthase during preoperative pelvic radiotherapy for locally advanced rectal carcinoma: long-term outcome. Int J Radiat Oncol Biol Phys 2011;79:670-6. [Crossref] [PubMed]
- Li XY, Liu L, Xie XM, et al. The role of raltitrexed/cisplatin with concurrent radiation therapy in treating advanced cervical cancer. Eur Rev Med Pharmacol Sci 2014;18:3491-6. [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Lopez L, Montenegro PC. Cardiotoxicity of 5-fluorouracil in patients with gastrointestinal cancer. J Clin Oncol 2012;30:689. [Crossref]
- Sun X, Han S, Gu F, et al. A Retrospective Comparison of Taxane and Fluorouracil-based Chemoradiotherapy in Patients with Inoperable Esophageal Squamous Cell Carcinoma. J Cancer 2016;7:1066-73. [Crossref] [PubMed]
- Liu Y, Wu W, Hong W, et al. Raltitrexed-based chemotherapy for advanced colorectal cancer. Clin Res Hepatol Gastroenterol 2014;38:219-25. [Crossref] [PubMed]
- Deboever G, Hiltrop N, Cool M, et al. Alternative treatment options in colorectal cancer patients with 5-fluorouracil- or capecitabine-induced cardiotoxicity. Clin Colorectal Cancer 2013;12:8-14. [Crossref] [PubMed]
- Kelly C, Bhuva N, Harrison M, et al. Use of raltitrexed as an alternative to 5-fluorouracil and capecitabine in cancer patients with cardiac history. Eur J Cancer 2013;49:2303-10. [Crossref] [PubMed]
- Ransom D, Wilson K, Fournier M, et al. Final results of Australasian Gastrointestinal Trials Group ARCTIC study: an audit of raltitrexed for patients with cardiac toxicity induced by fluoropyrimidines. Ann Oncol 2014;25:117-21. [Crossref] [PubMed]


